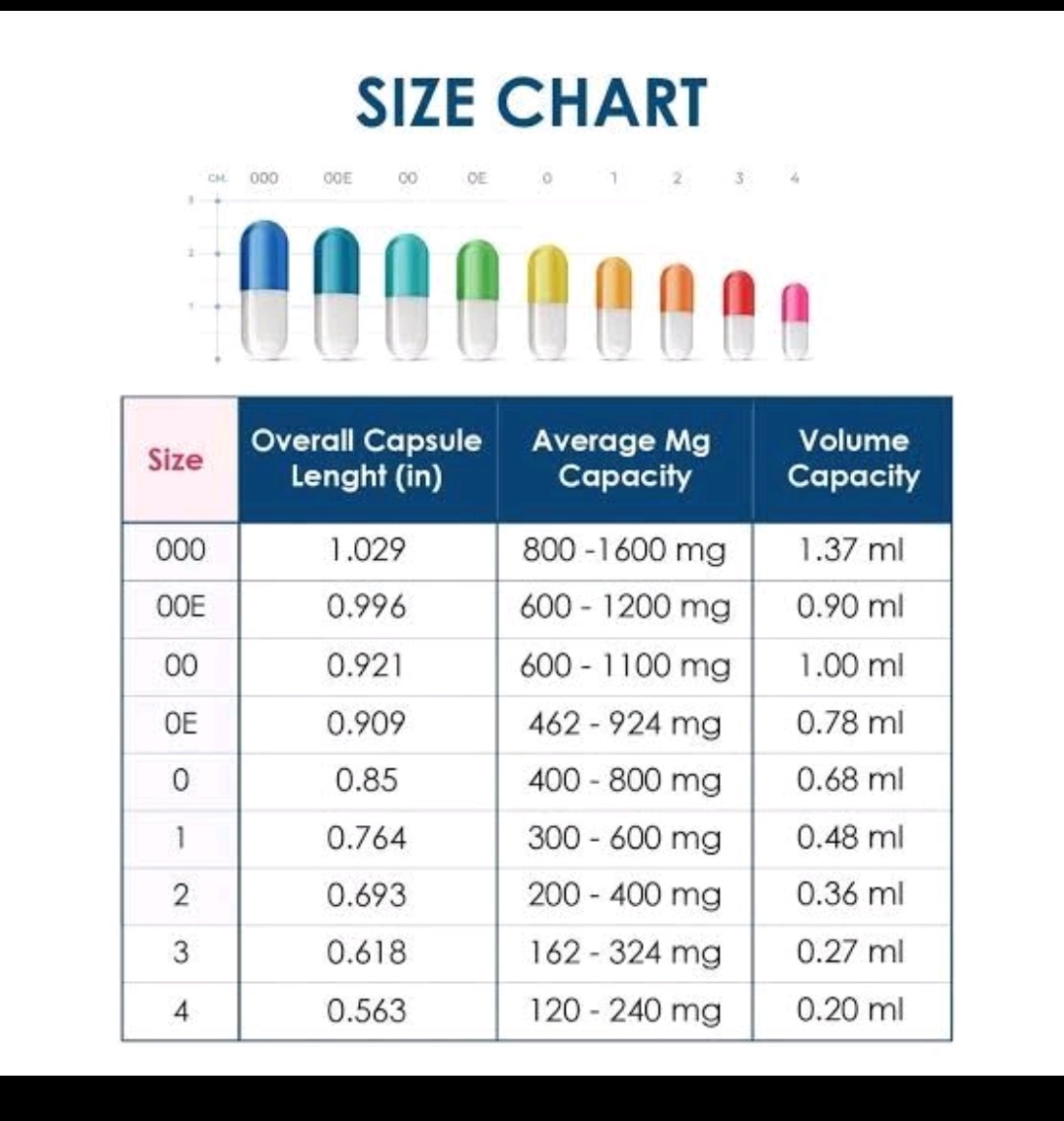
Test in Pharma
Description, Identification, Assay, DT, Dissolution, Impurities. Related Substance, Friability, Hardness, Average Weight, Content Uniformity, Microbial Limits, pH, Viscosity, sterility,.
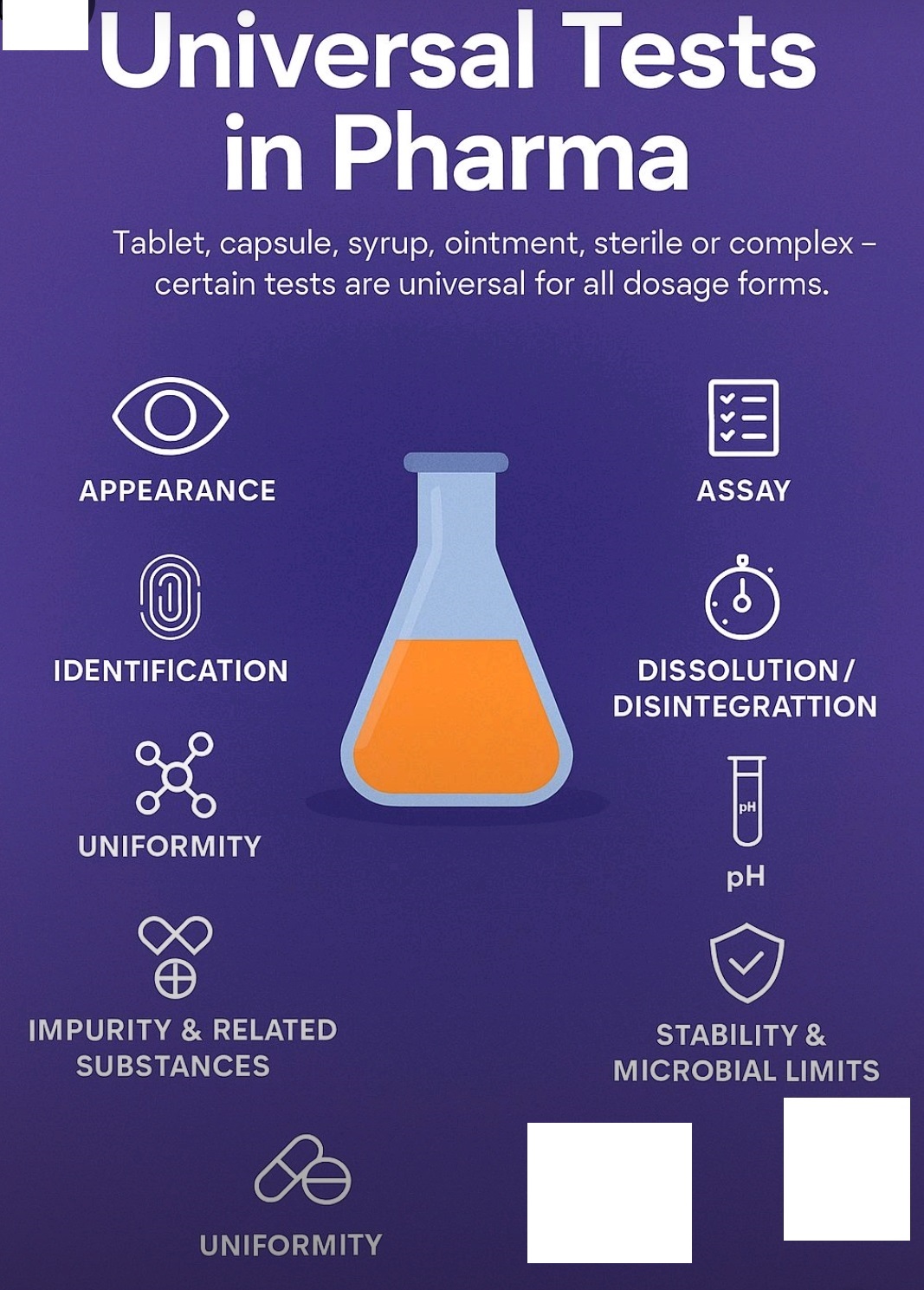

Pharma Definition/Abbreviation, Pharma Beginners
Description, Identification, Assay, DT, Dissolution, Impurities. Related Substance, Friability, Hardness, Average Weight, Content Uniformity, Microbial Limits, pH, Viscosity, sterility,.


A Validation Master Plan (VMP) in pharma is a high-level strategic document outlining the entire facility’s approach to validation, serving as a roadmap to ensure all equipment, systems, facilities, and processes consistently meet regulatory standards (FDA, EMA) and produce quality products. It defines validation scope, schedules, responsibilities, and documentation, integrating activities like IQ/OQ/PQ, process validation, and cleaning validation into a unified, risk-based framework for GMP compliance and audit readiness.

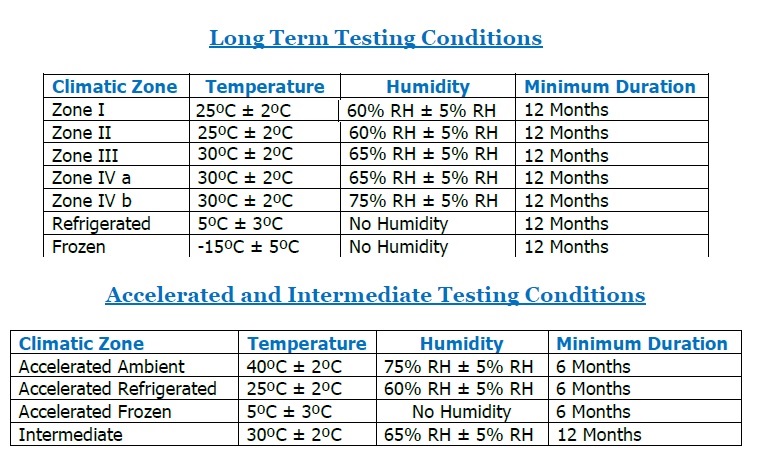
Stability Testing Conditions
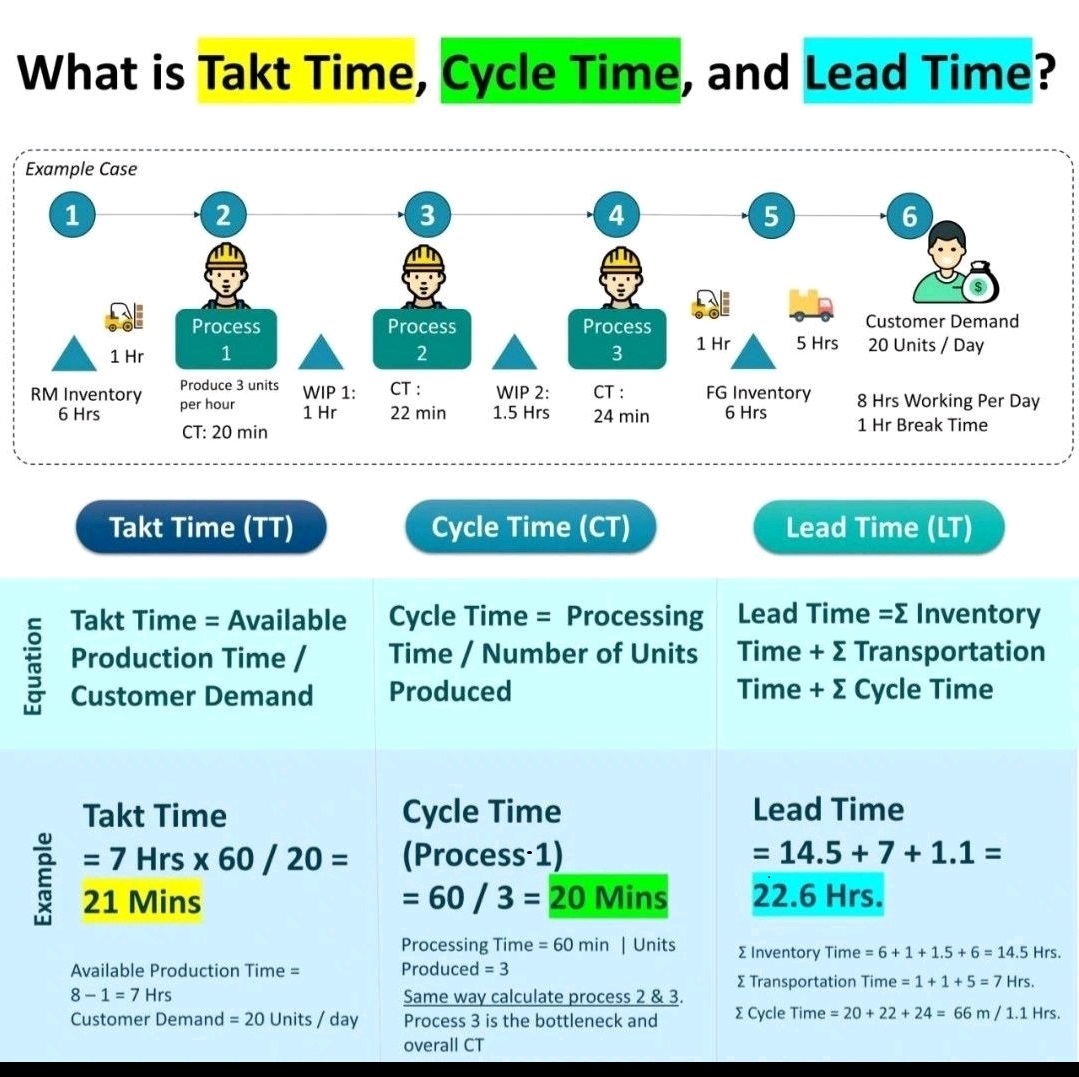
Inventory Management-ROP

FIFO/LIFO/CIFO/DEFO/EFO
(first in,first out/ last in,last out)
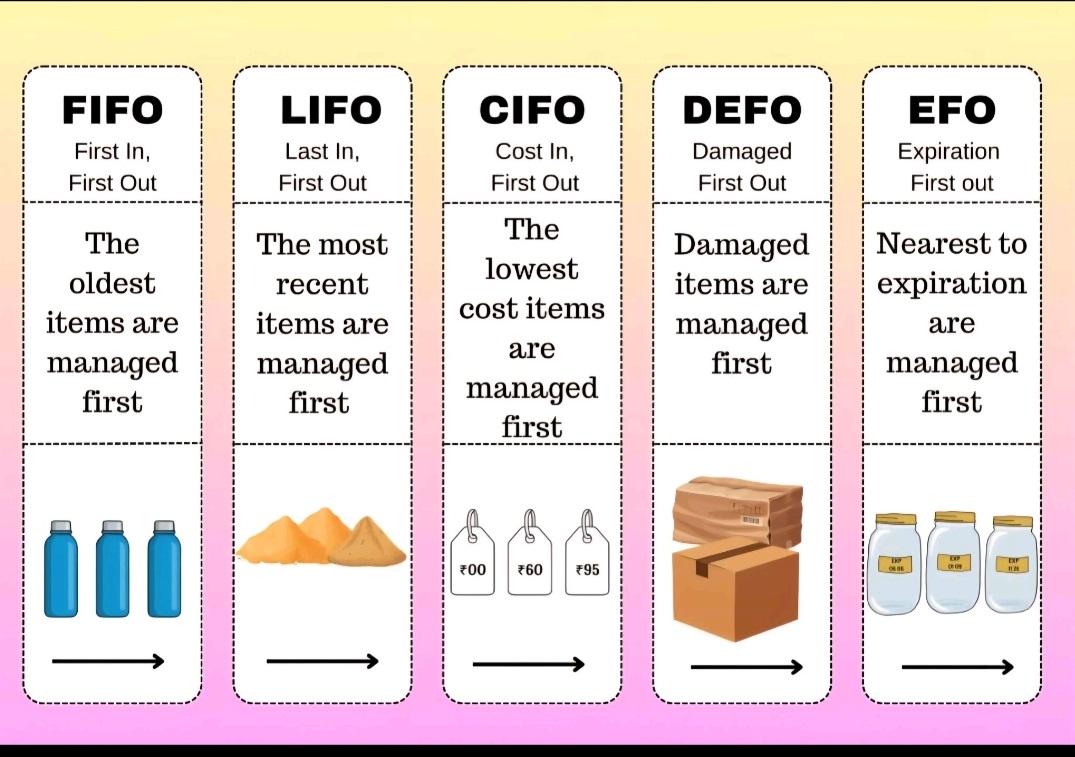
ABC Inventory- High cost,Moderate cost, Low cost.

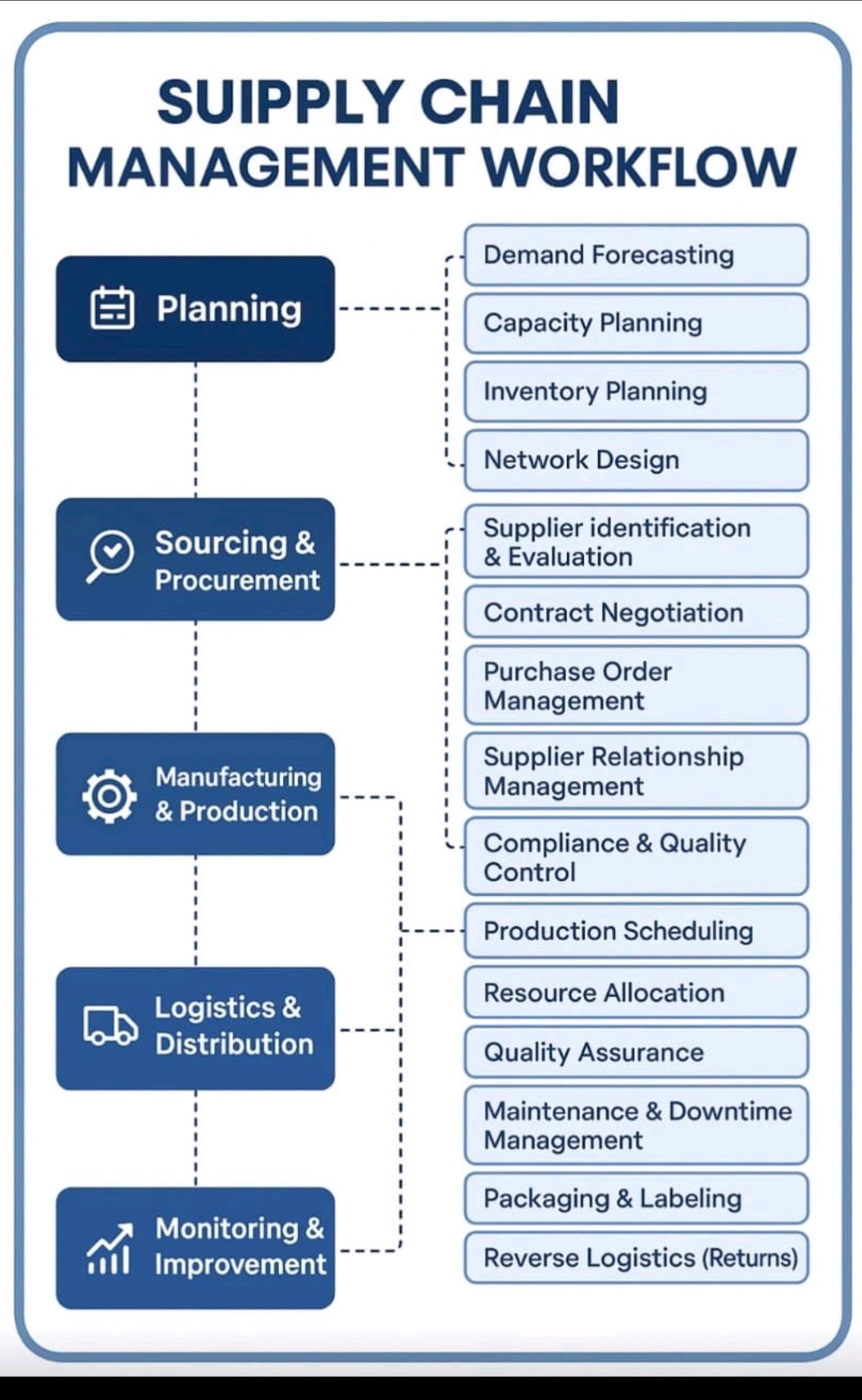
Supply Chain Management Workflow
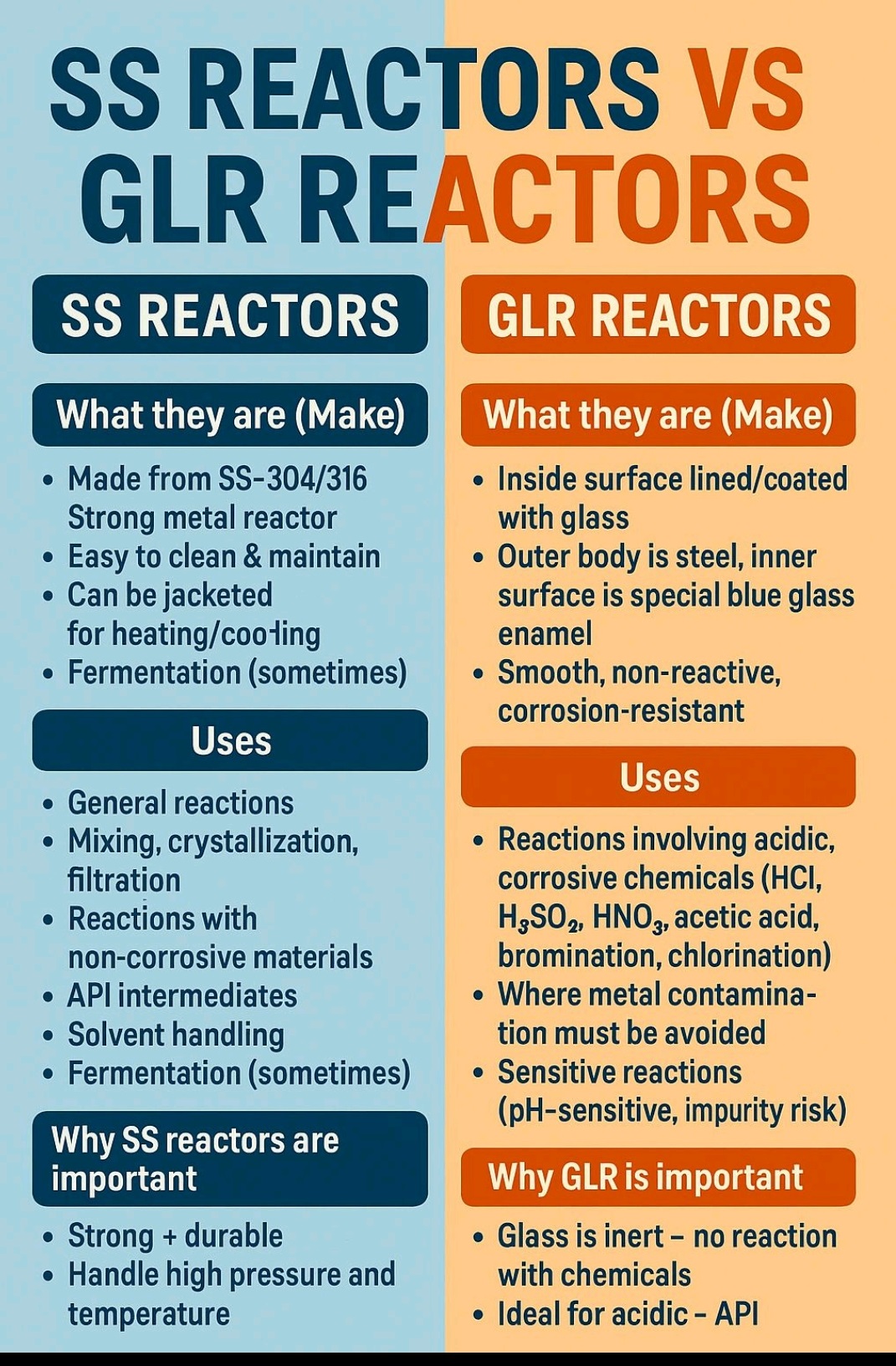
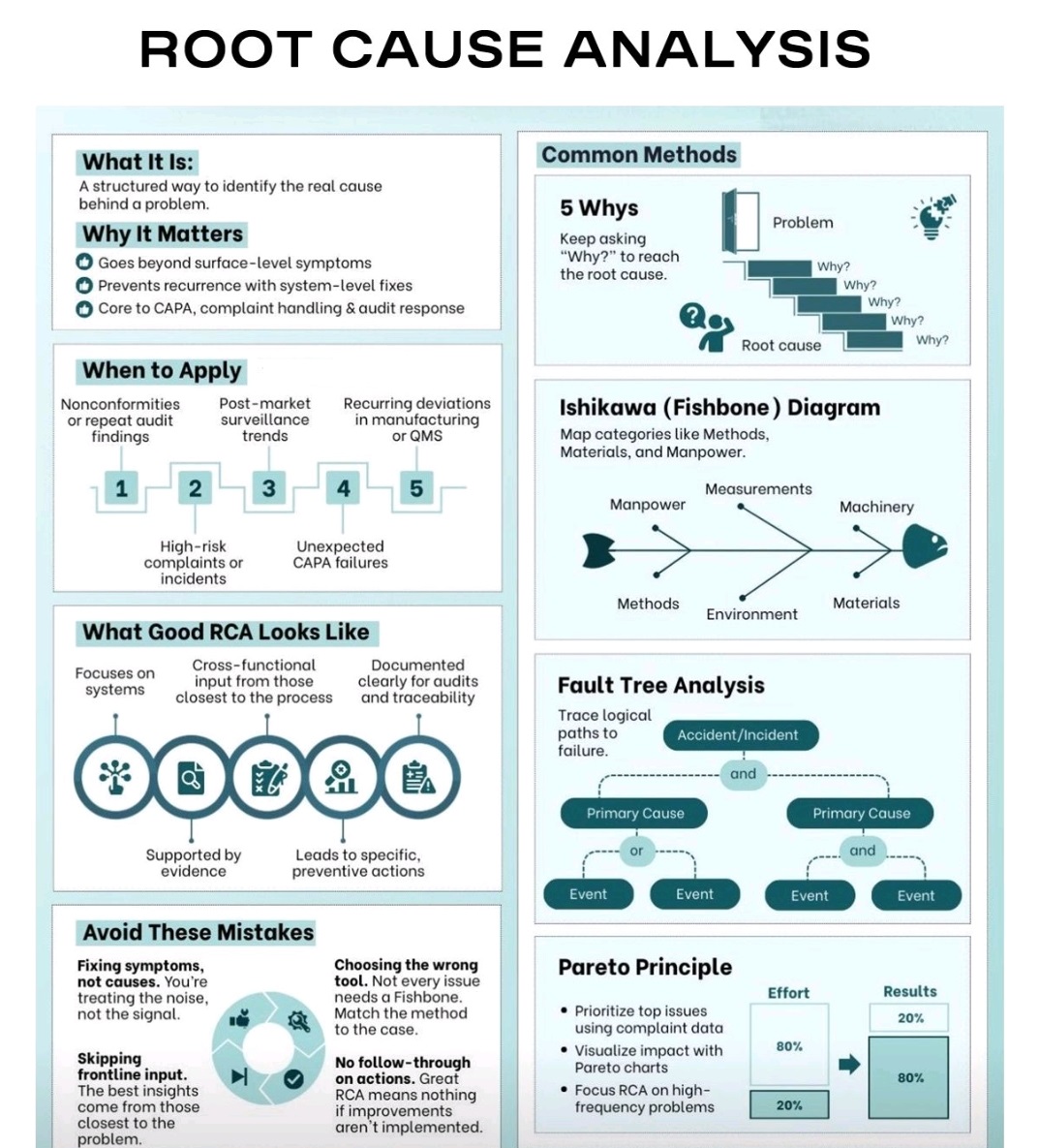
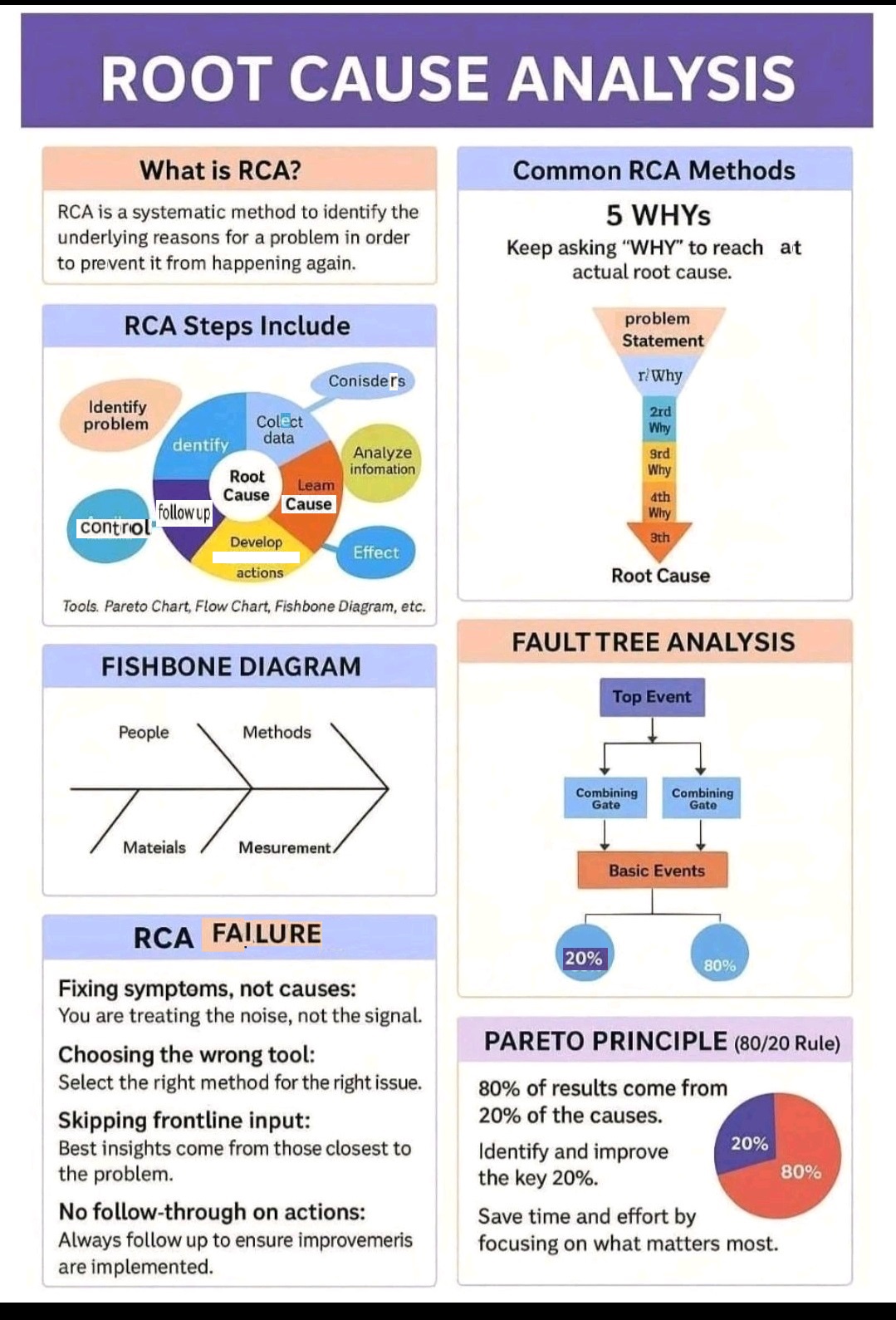
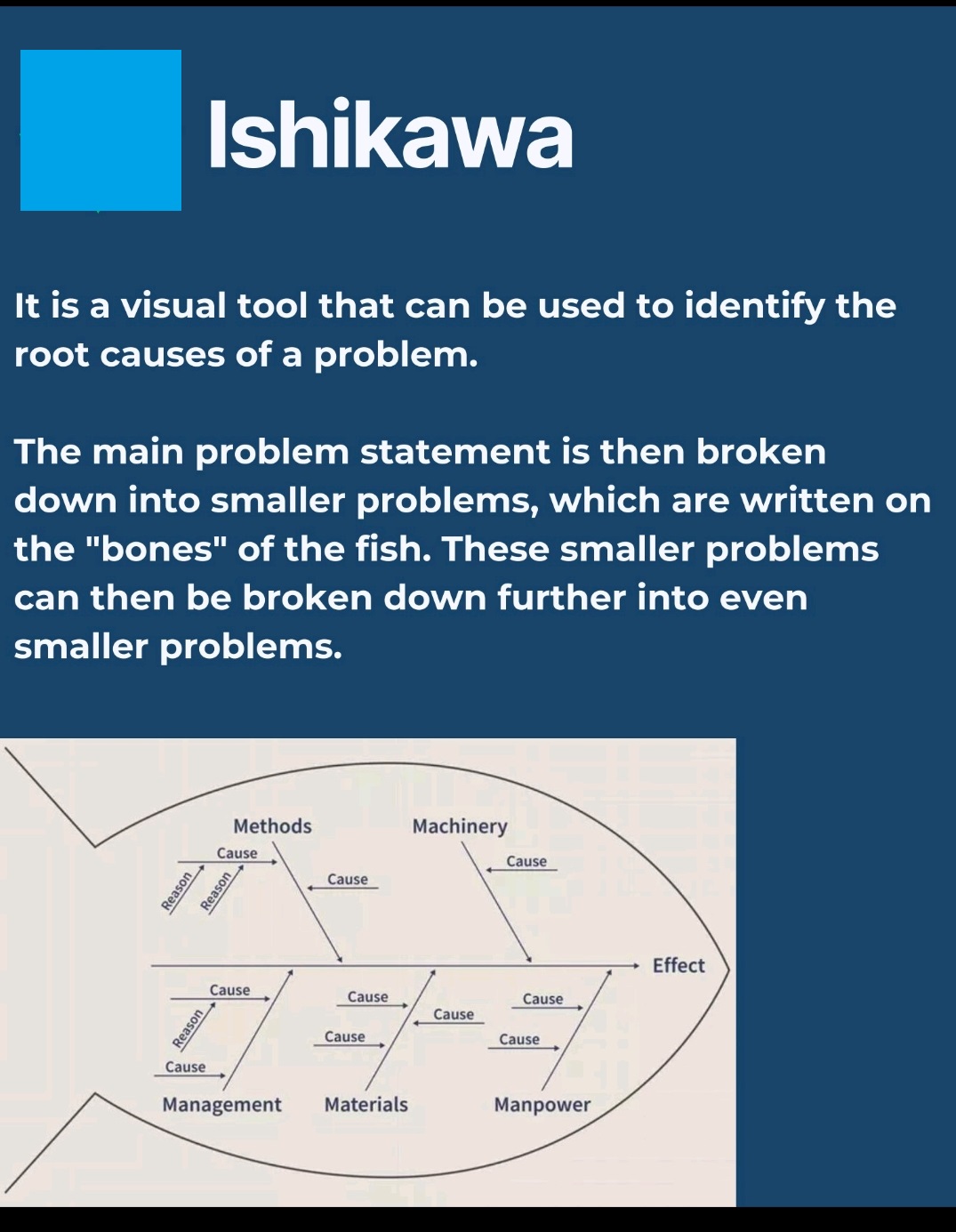
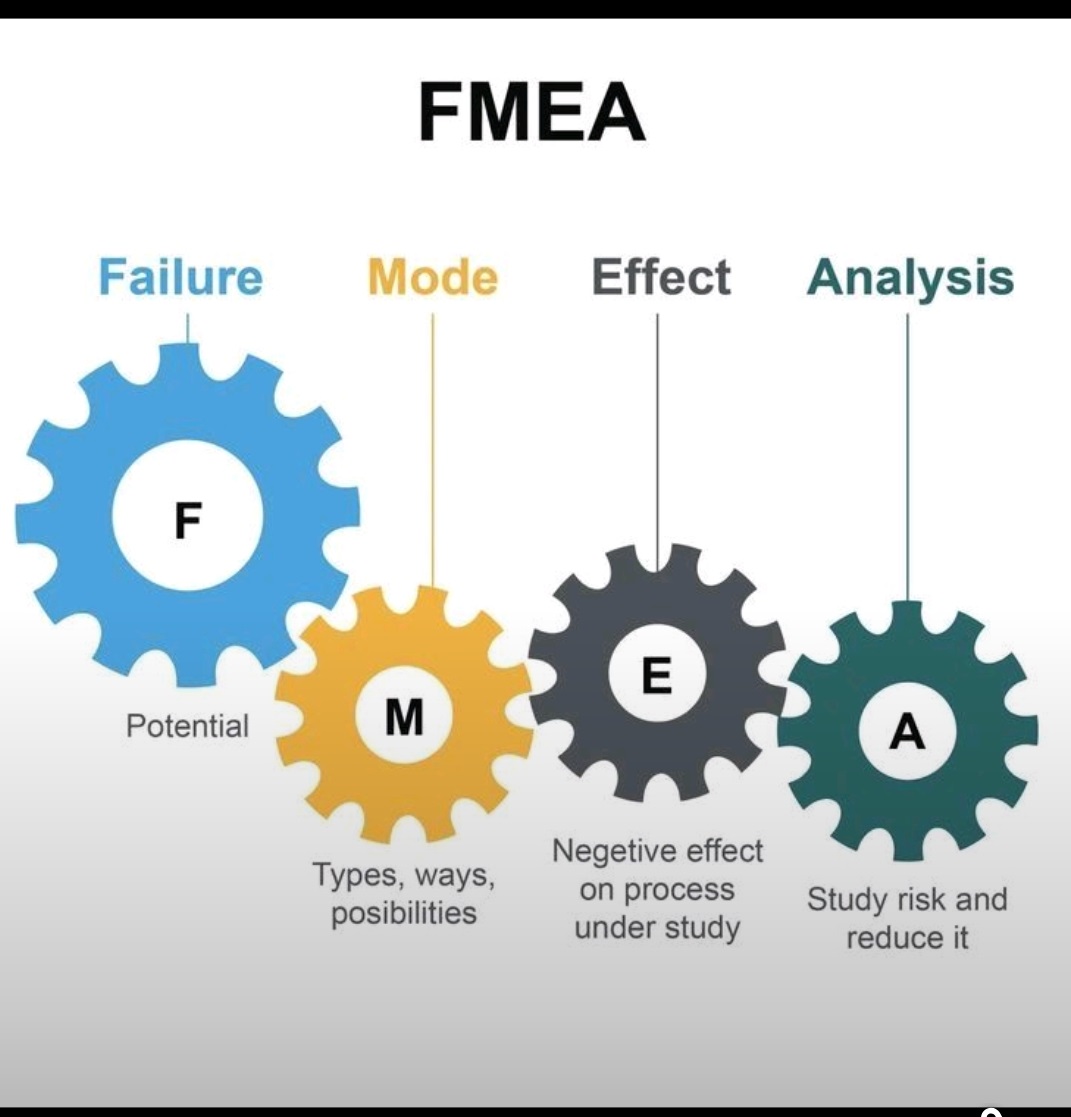
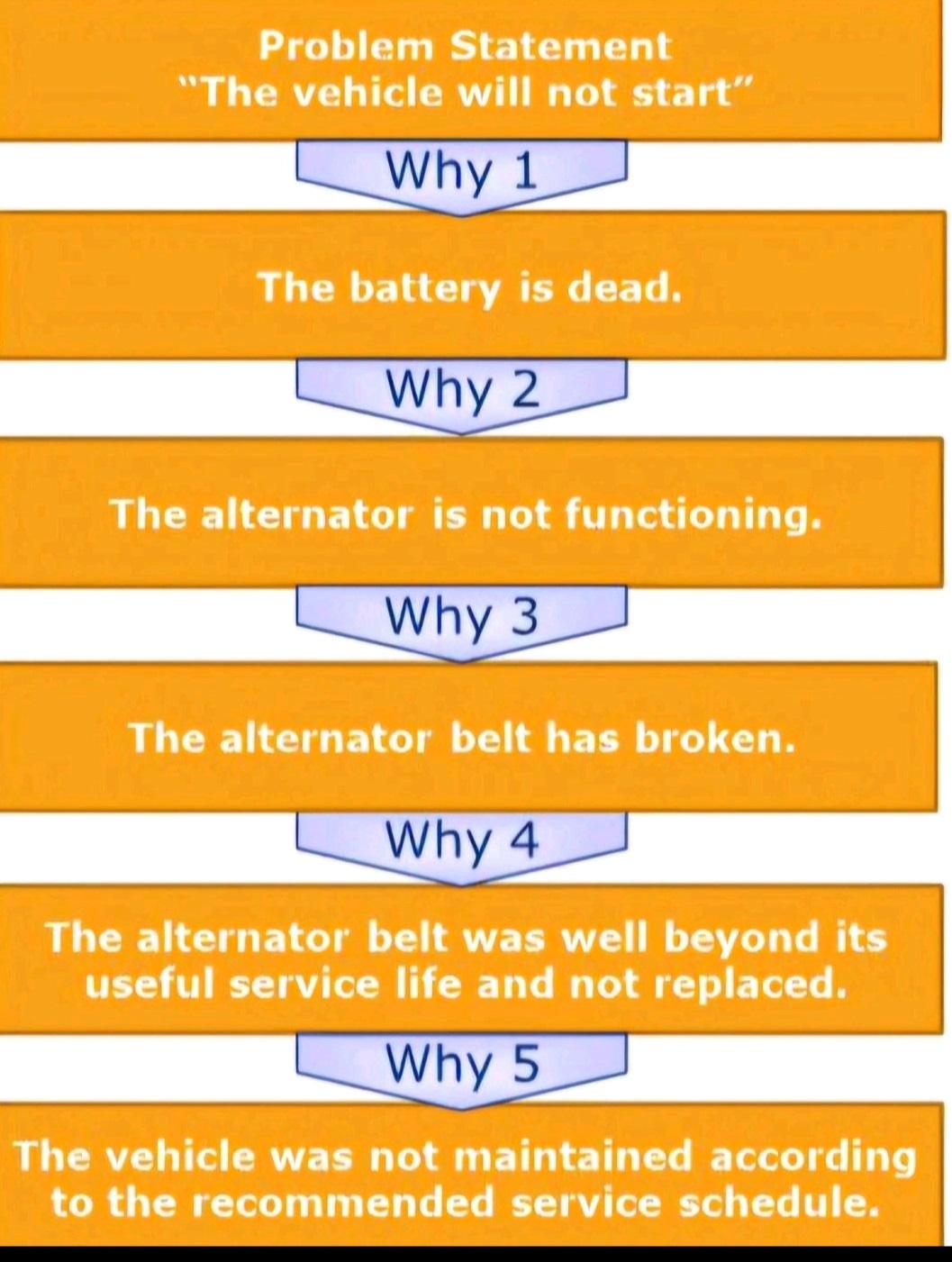
What is Root Cause analysis?
Root cause analysis (RCA) is a systematic problem-solving method used to identify the underlying cause of a problem or incident, rather than just treating its symptoms.
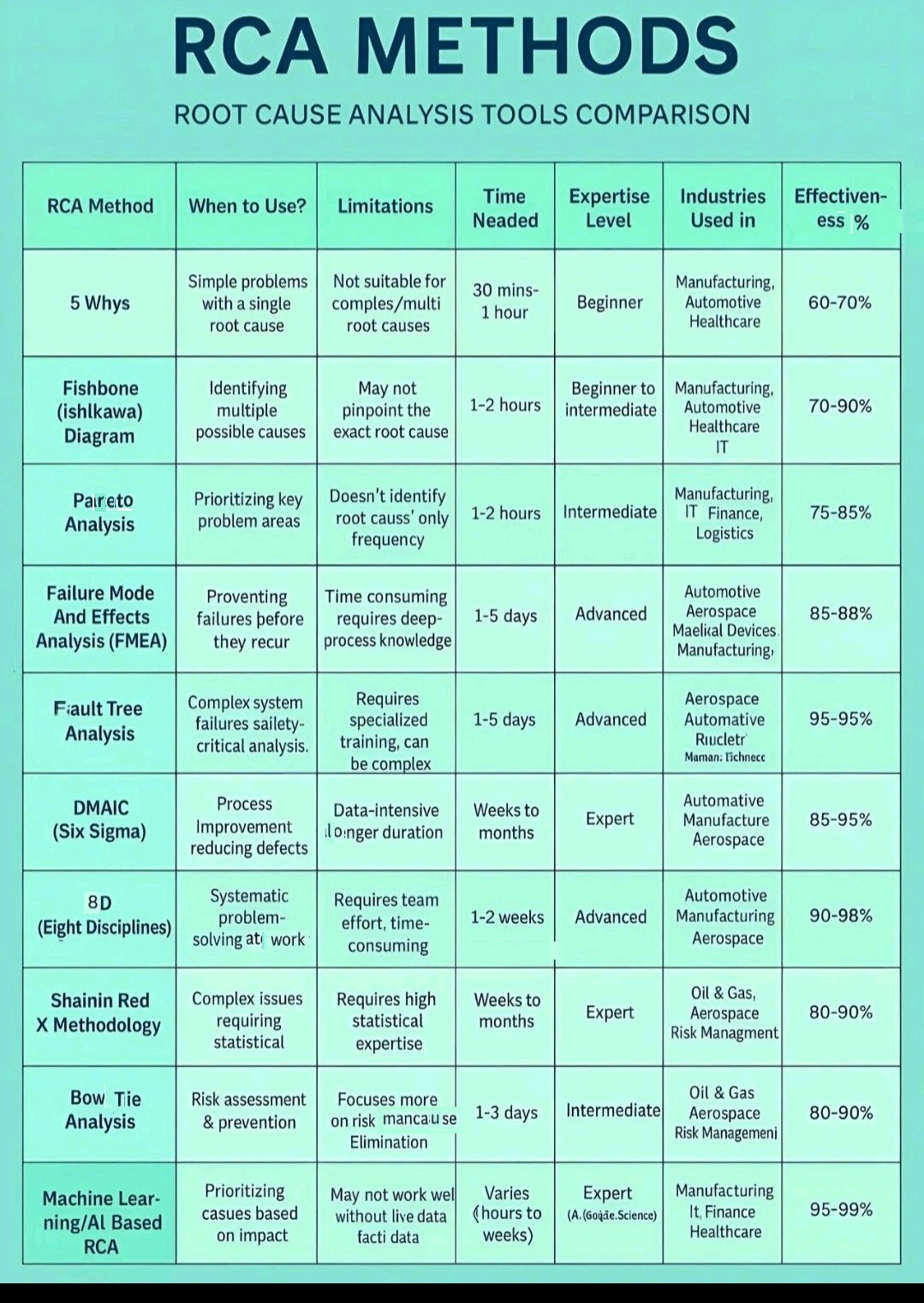
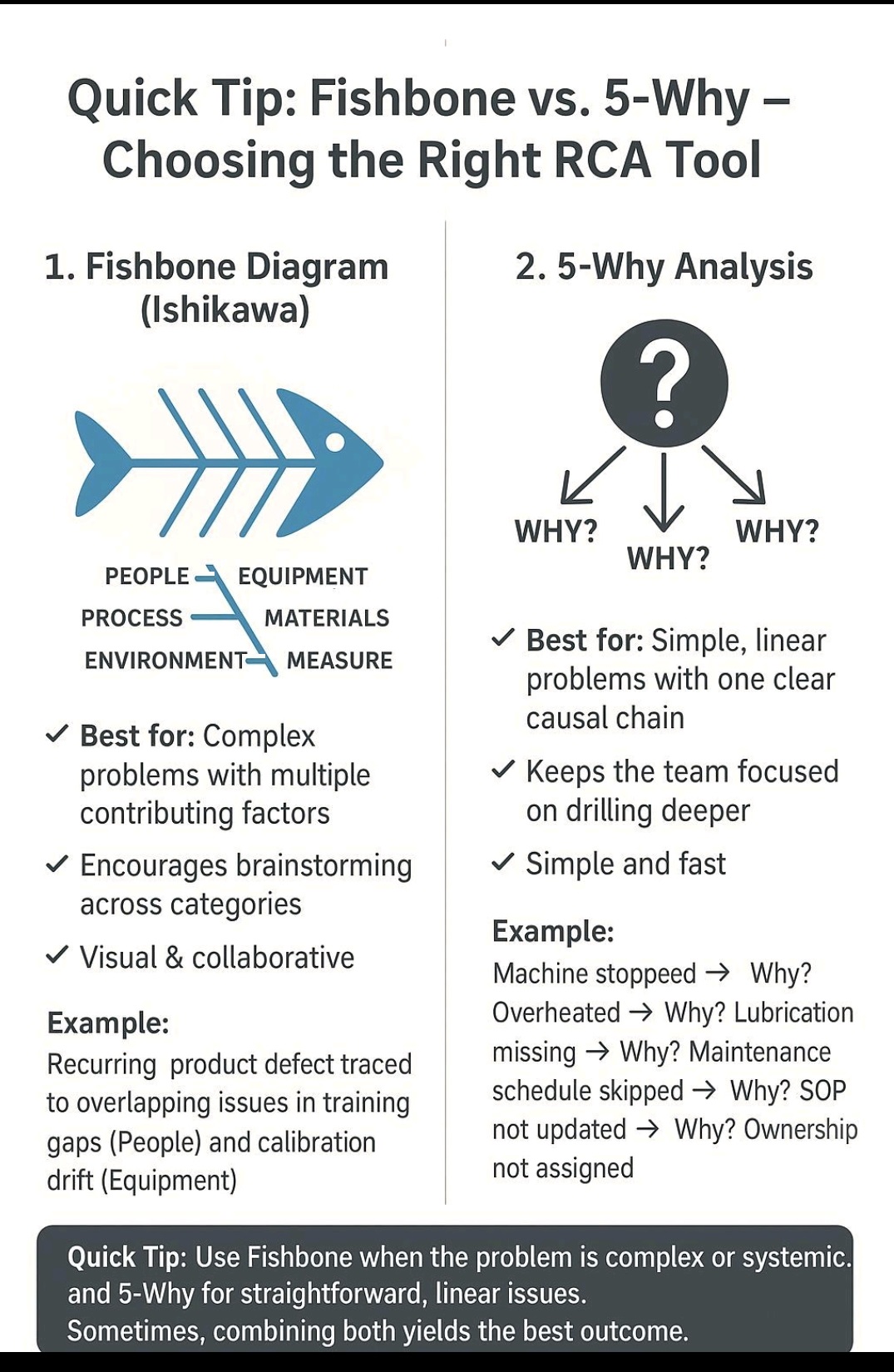
5 Whys, Fishbone Diagram-Ishikawa, Pareto analysis, Fault tree analysis (FTA)