ICH Guideline-Quality


ICH Guidelines for Pharmaceuticals substance and products



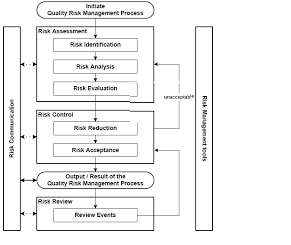
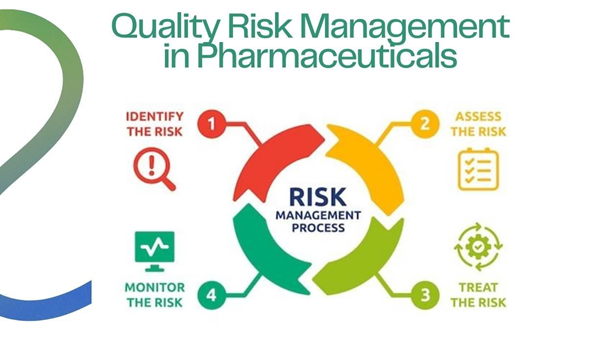
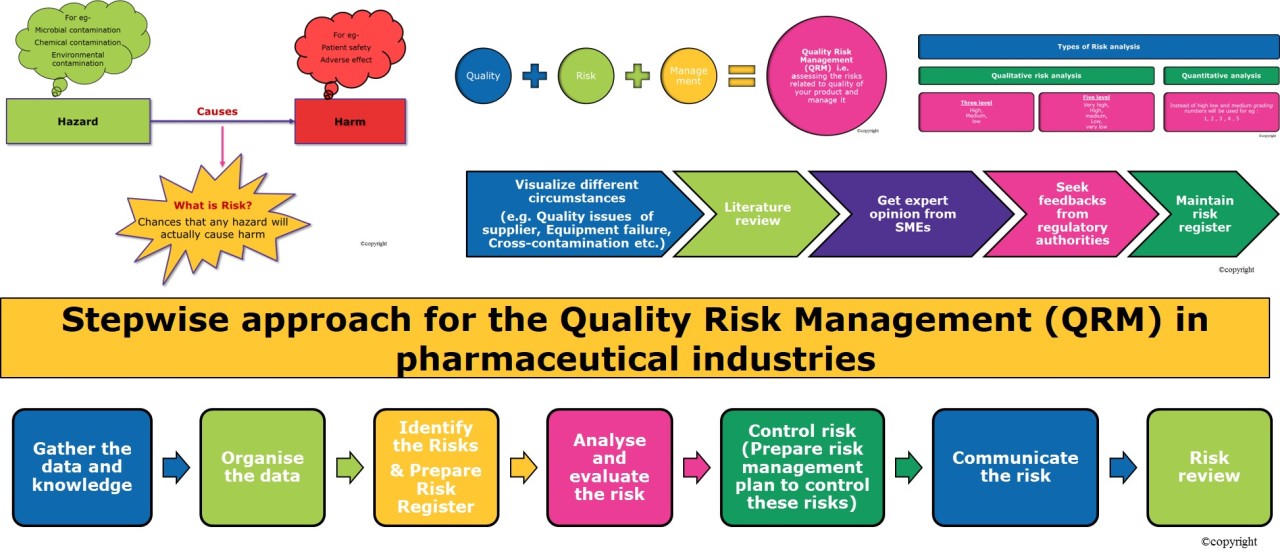
Quality Risk Management (QRM) is a systematic, science-based approach to identifying, assessing, controlling, communicating, and reviewing risks to product quality throughout its lifecycle. The goal is to ensure product safety and quality.
Key components of the QRM process
Benefits of Quality Risk Management
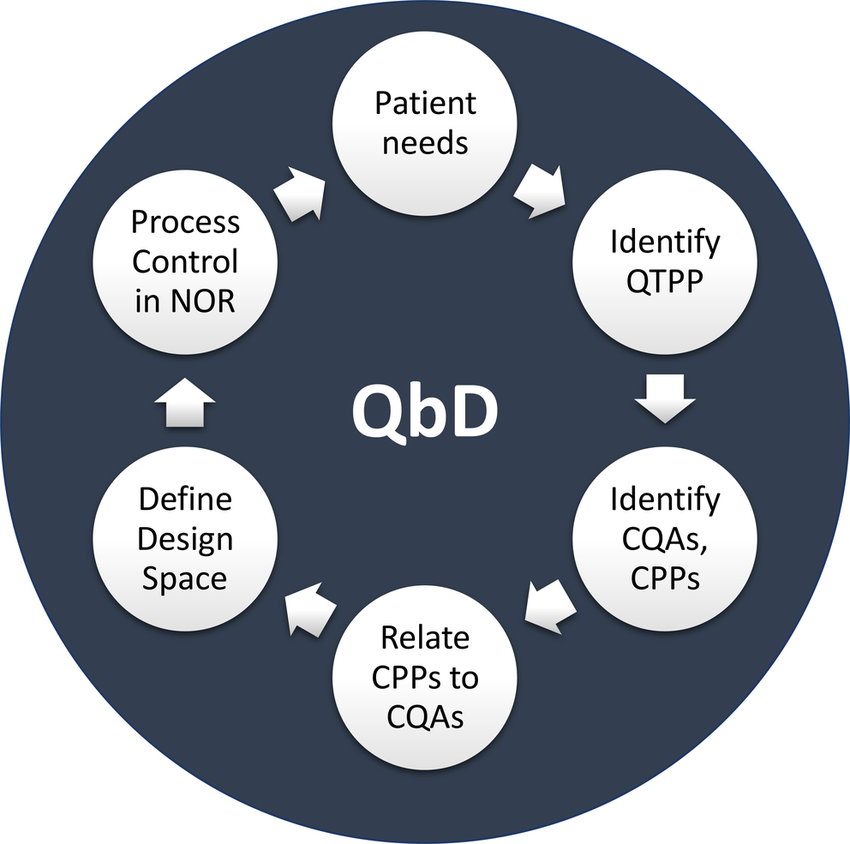
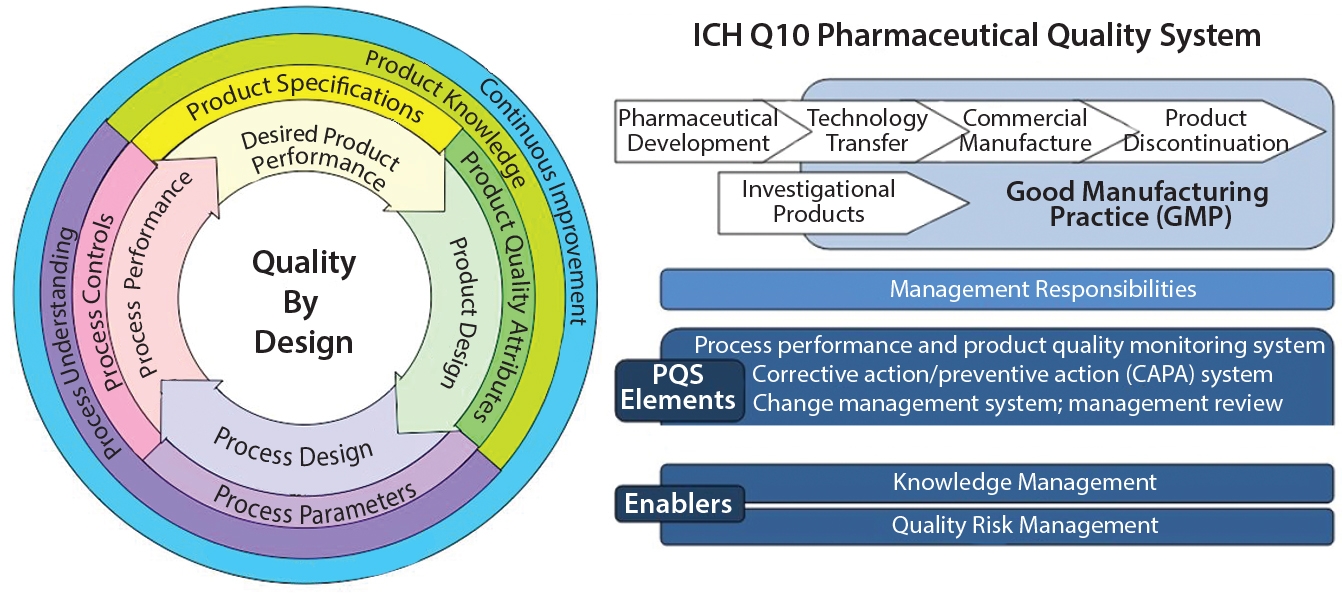
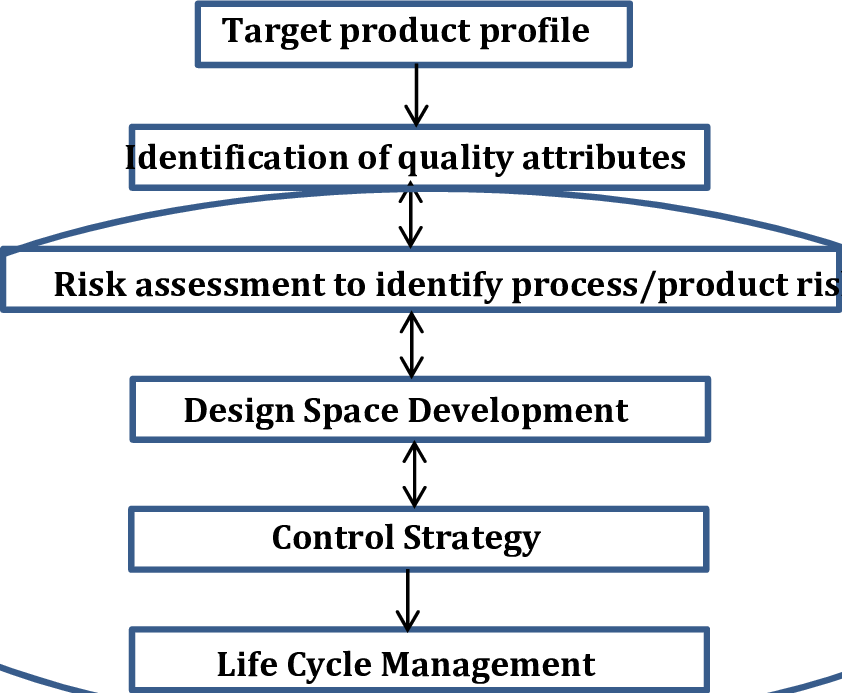
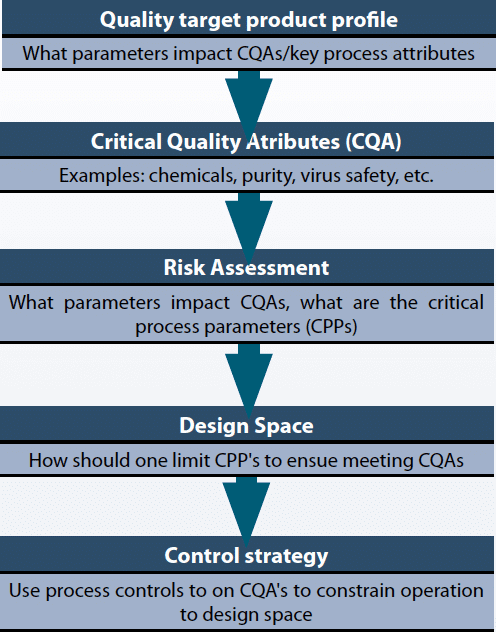
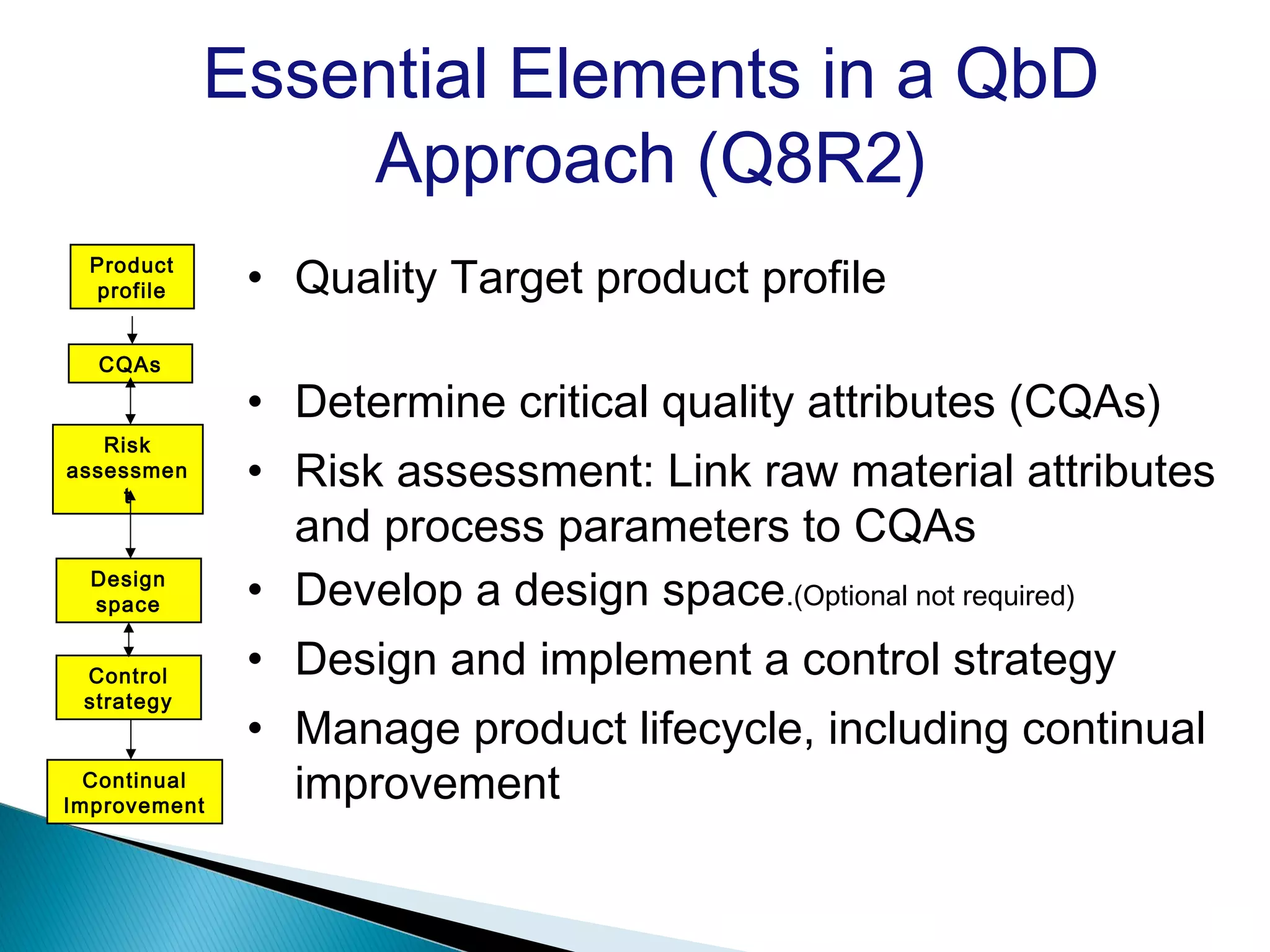
Quality by Design (QbD) is a systematic approach to product development that builds quality in from the start by focusing on a deep understanding of the product and its manufacturing process. It uses a science-based, risk-management approach to identify critical quality attributes (CQAs) and critical process parameters (CPPs), establishing a “design space” and control strategy to consistently meet quality targets throughout the product lifecycle.
Key principles and steps :-
Here’s a list of ICH Quality Guidelines along with the direct hyperlinks to each guideline on the ICH website:
Note:- These links direct you to the official ICH website where you can access the full texts of each guideline. Each page contains the relevant guideline documents, details, and updates related to that particular quality standard.