Semiconductors
Intrinsic Semiconductors, Extrinsic Semiconductors, n-Type, p-Type, Silicon, Germanium,Doping.
![]()
All chemistry related articles
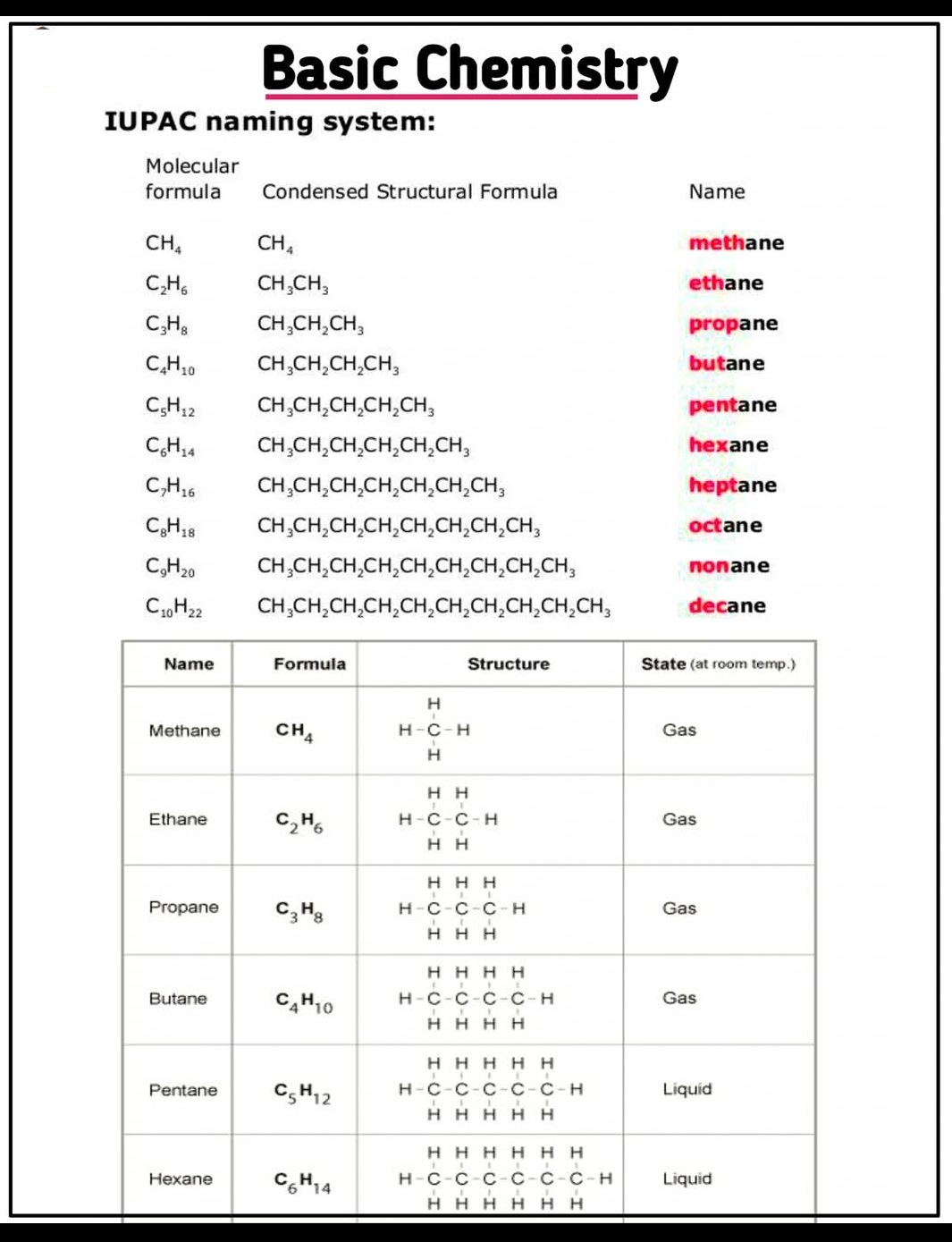
Intrinsic Semiconductors, Extrinsic Semiconductors, n-Type, p-Type, Silicon, Germanium,Doping.
![]()
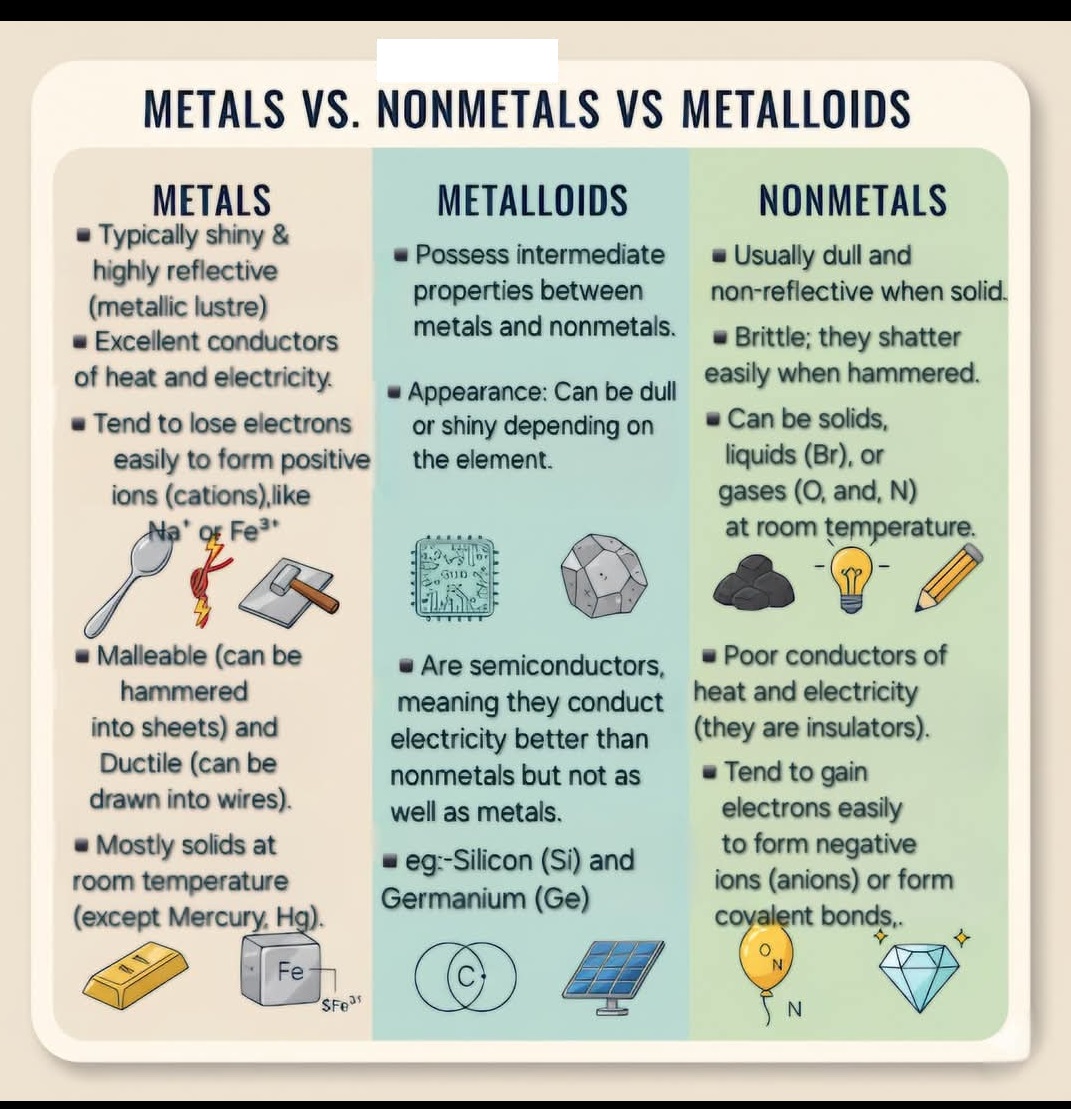
Properties of Metals, Non-Metals and Metalloids
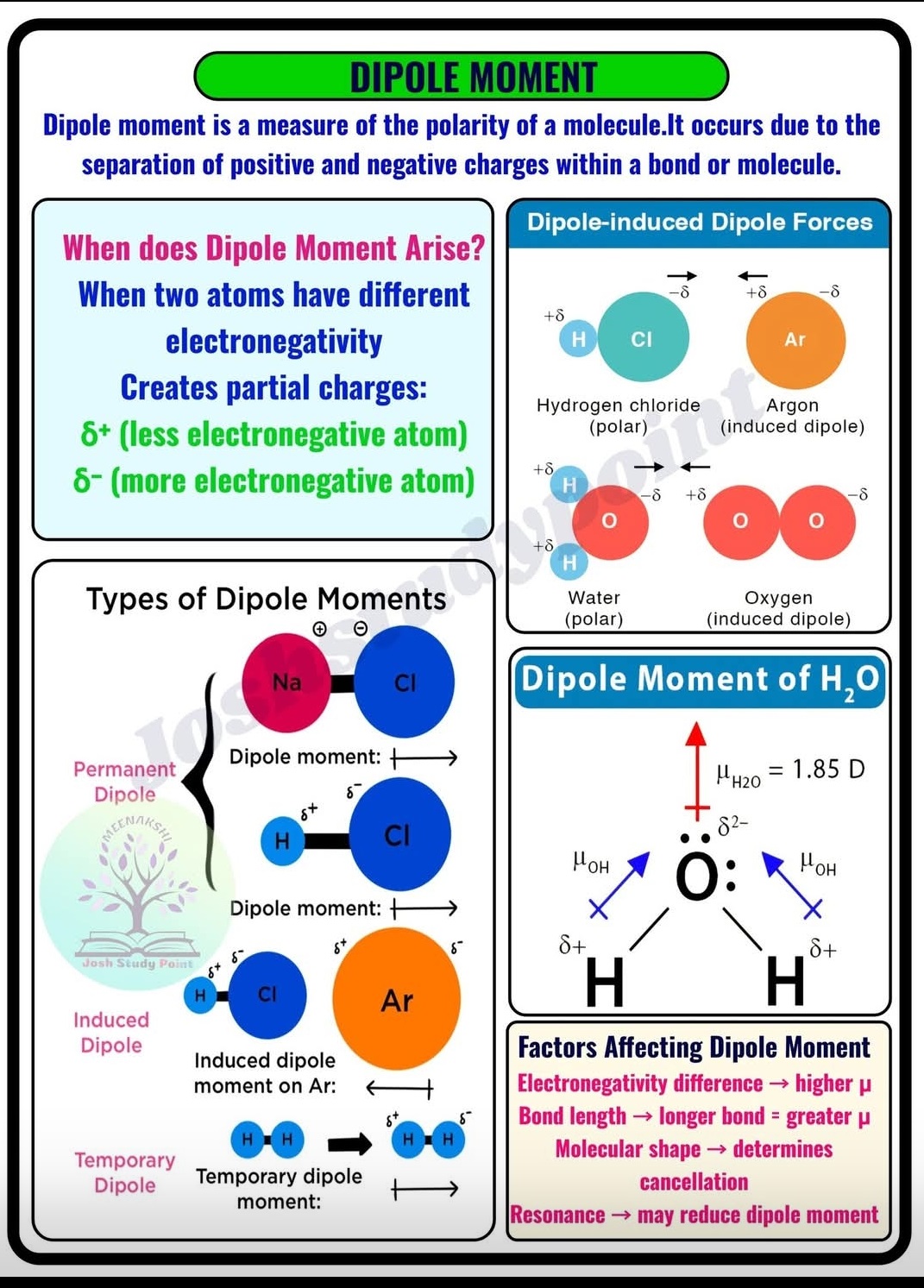
A molecule’s dipole moment is a measure of its polarity, representing the separation of positive and negative charges.
Alkaline earth metals are the six elements in Group 2 of the periodic table: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra). They are shiny, silvery-white, moderately reactive metals known for forming basic (alkaline) solutions in water and having two valence electrons, which they readily lose to form +2 ions, making them important in ionic compounds.
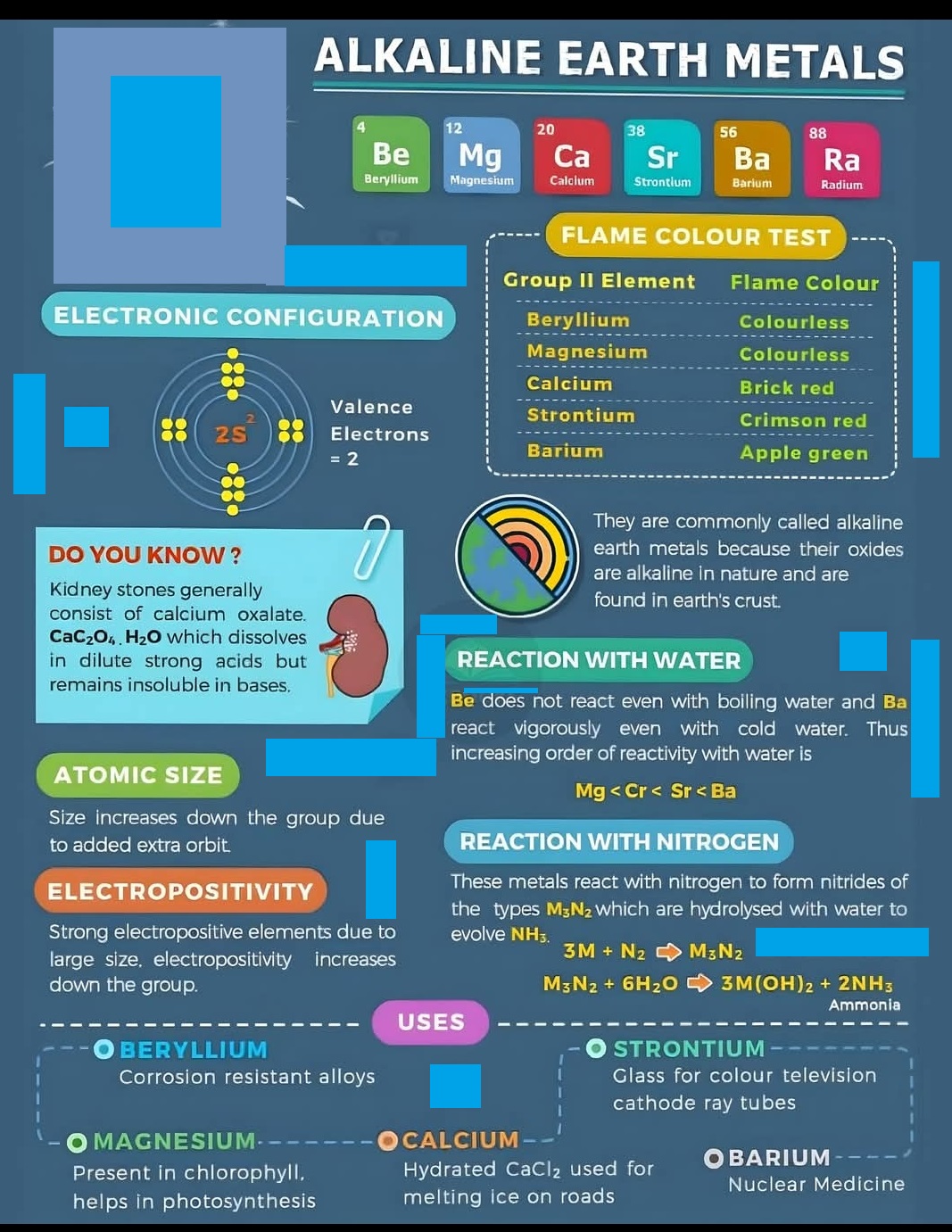
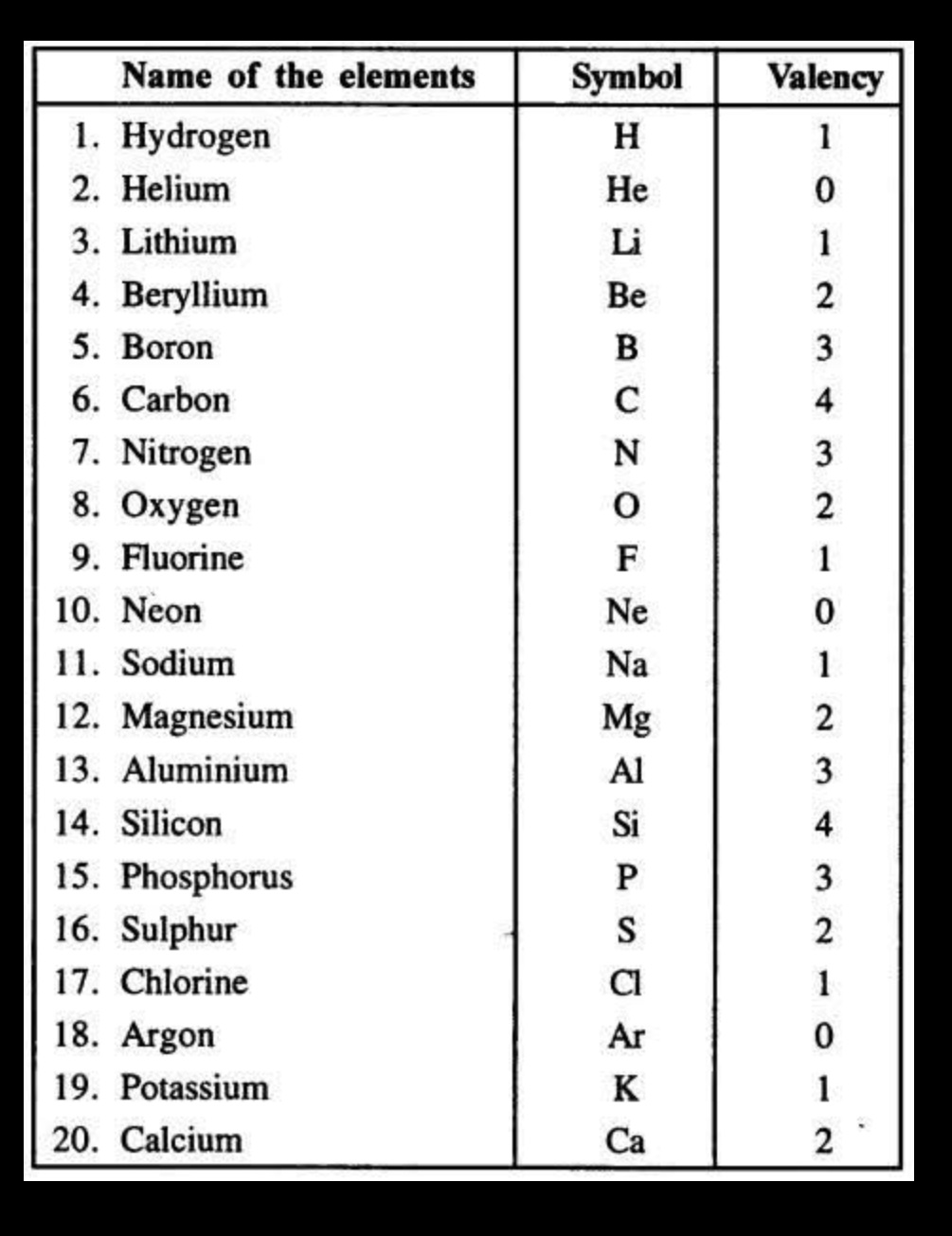
Valency is the combining capacity of an atom, referring to the number of electrons it can gain, lose, or share to form chemical bonds. It is determined by the number of electrons in an atom’s outermost shell (valence electrons) and its ability to achieve a stable electron configuration, often like that of a noble gas.
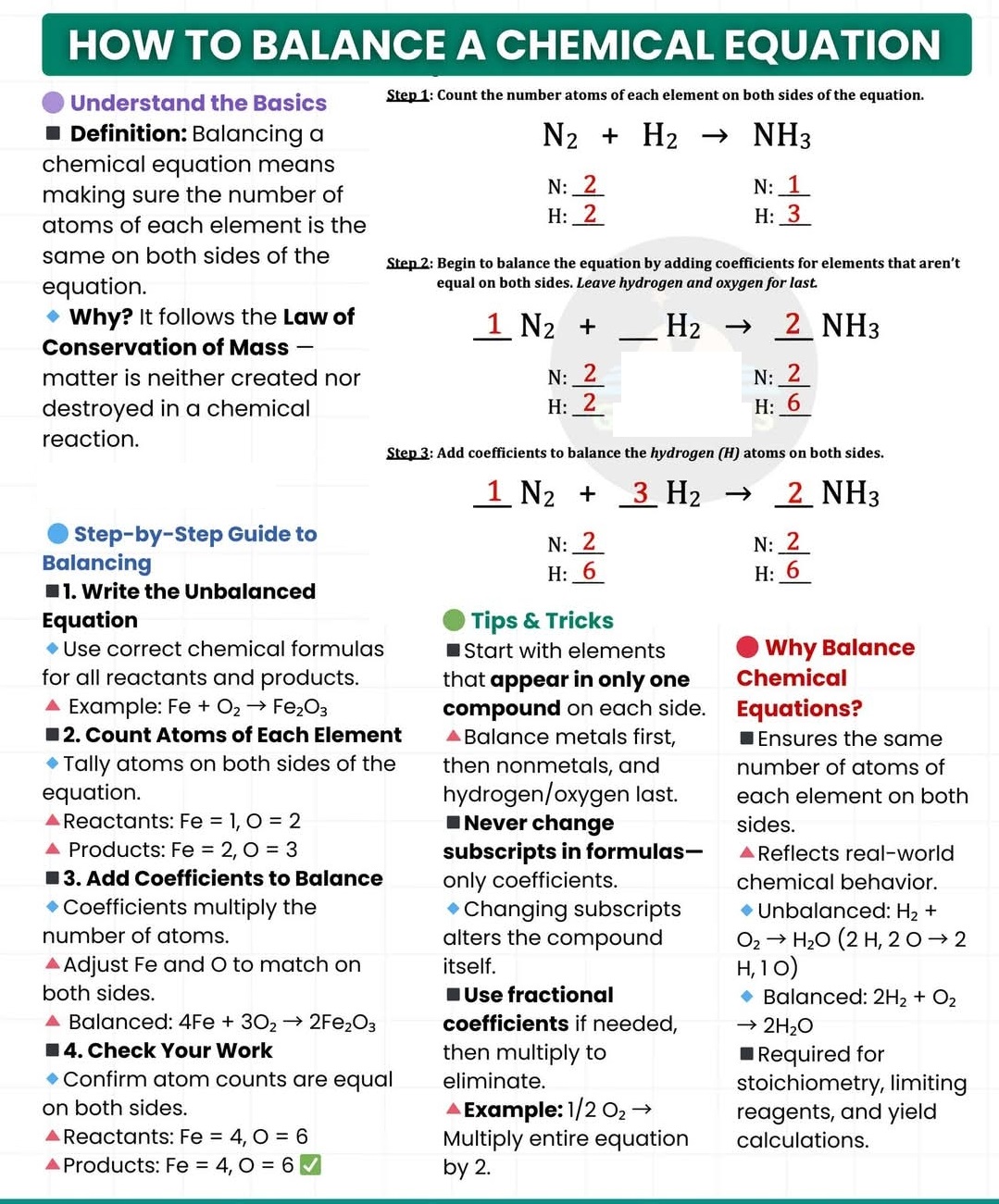
How to balance chemical reaction?
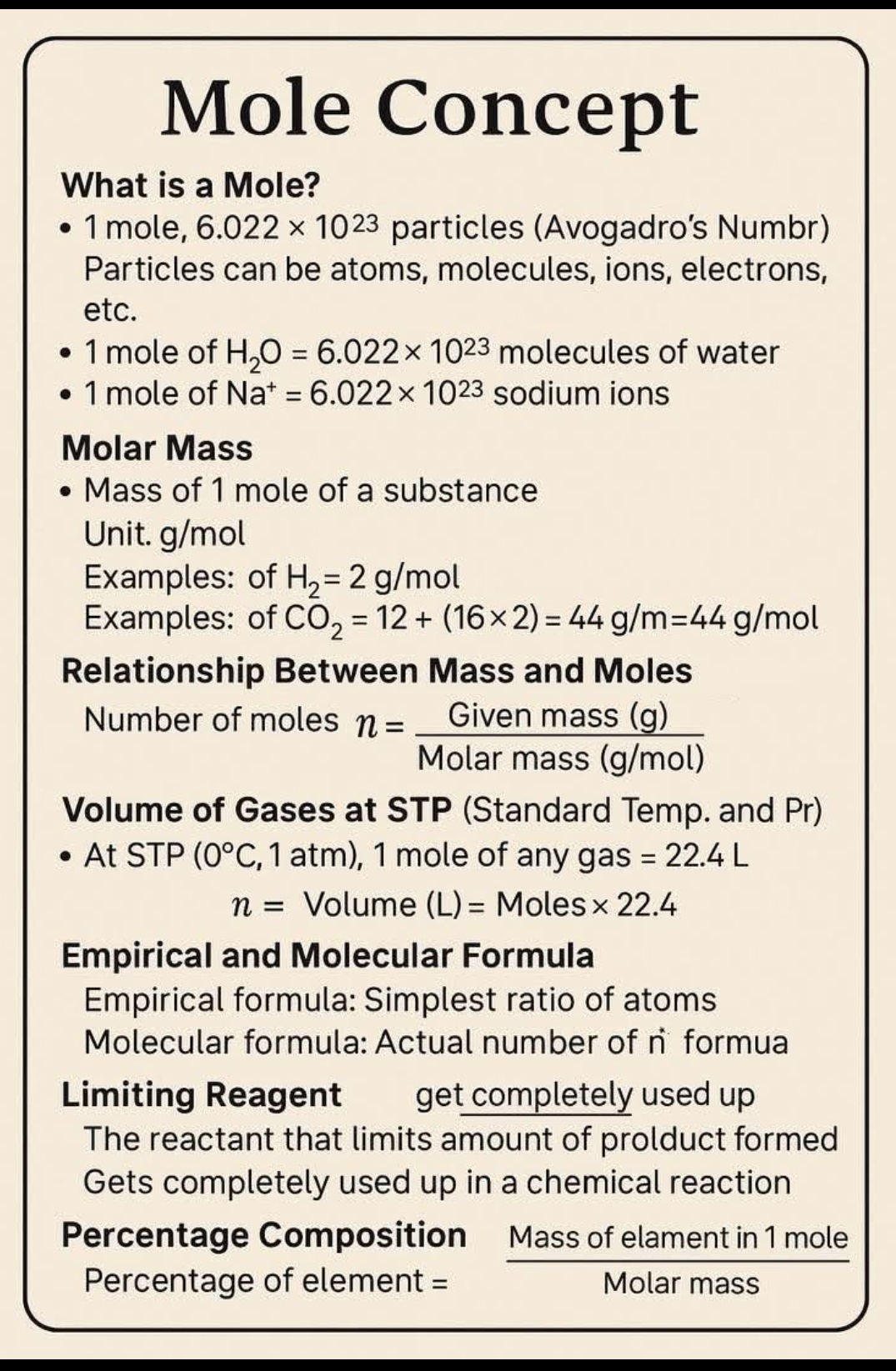
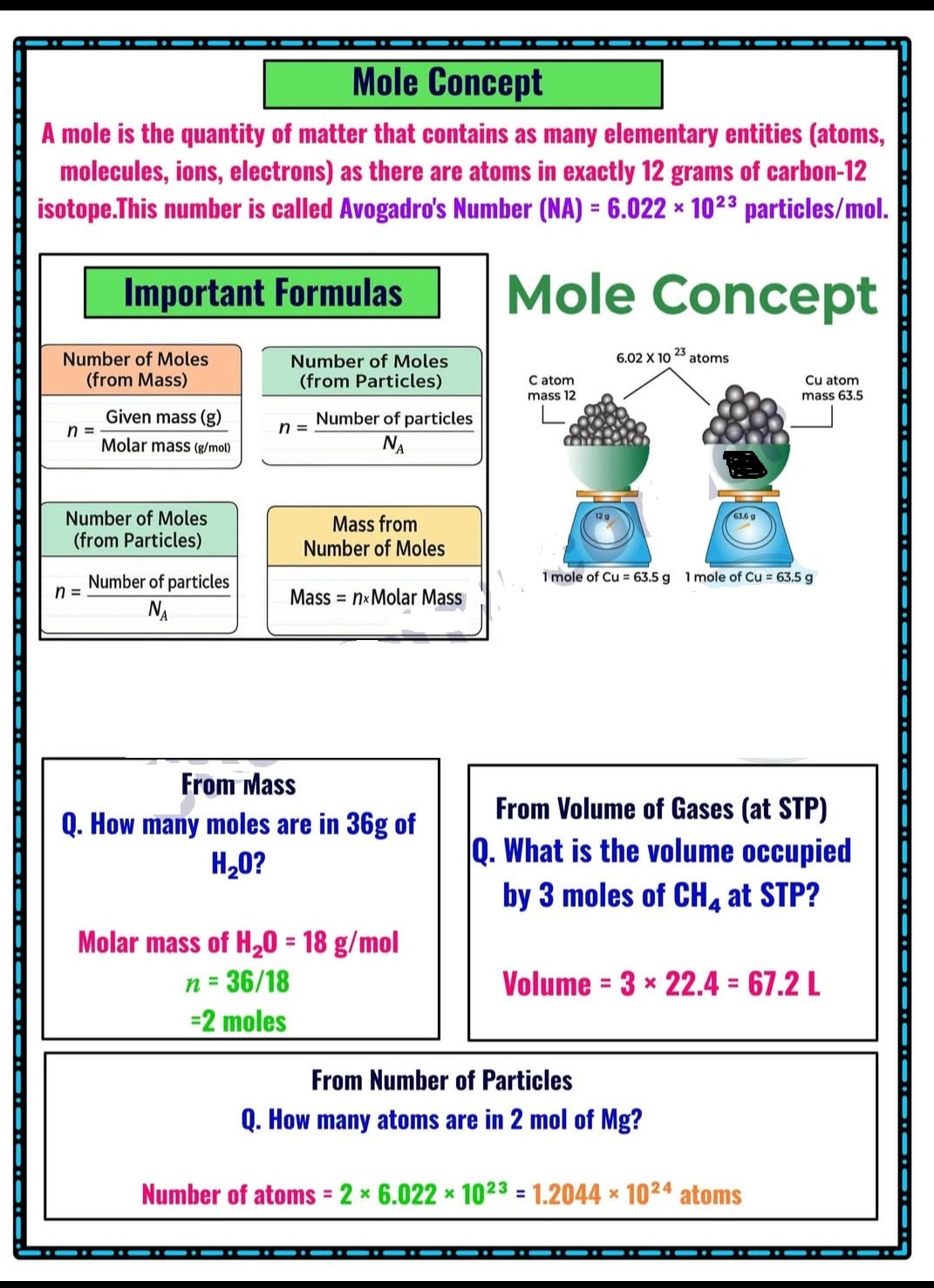
What is Mole, Molar Mass, volume of gases at STP, Empirical formula, Molecular Formula?
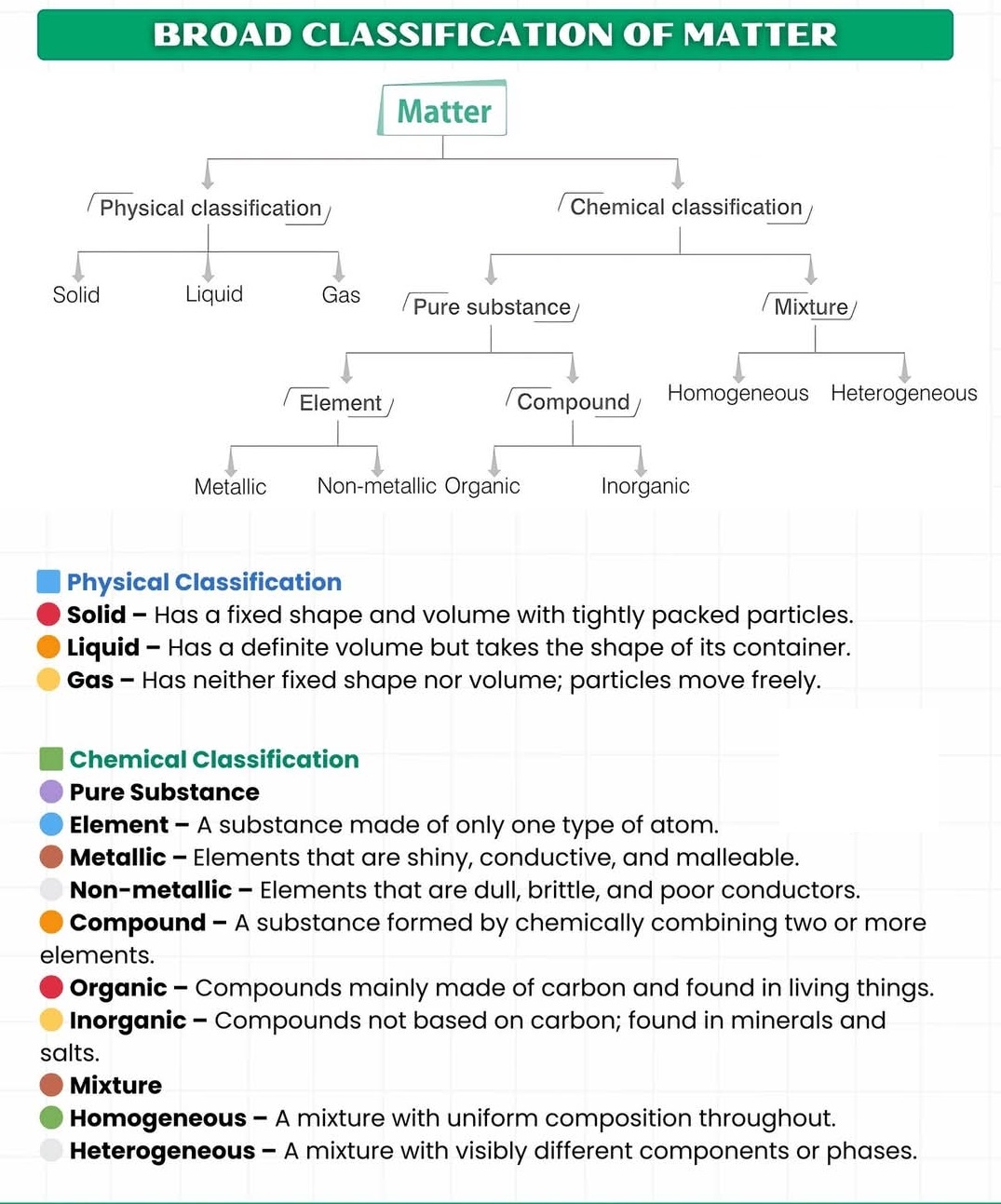
Classification of Matter (Element, compound, Mixture, Metallic, Non-Metallic,Organic,In-organic,Homogeneous,Heterogeneous)
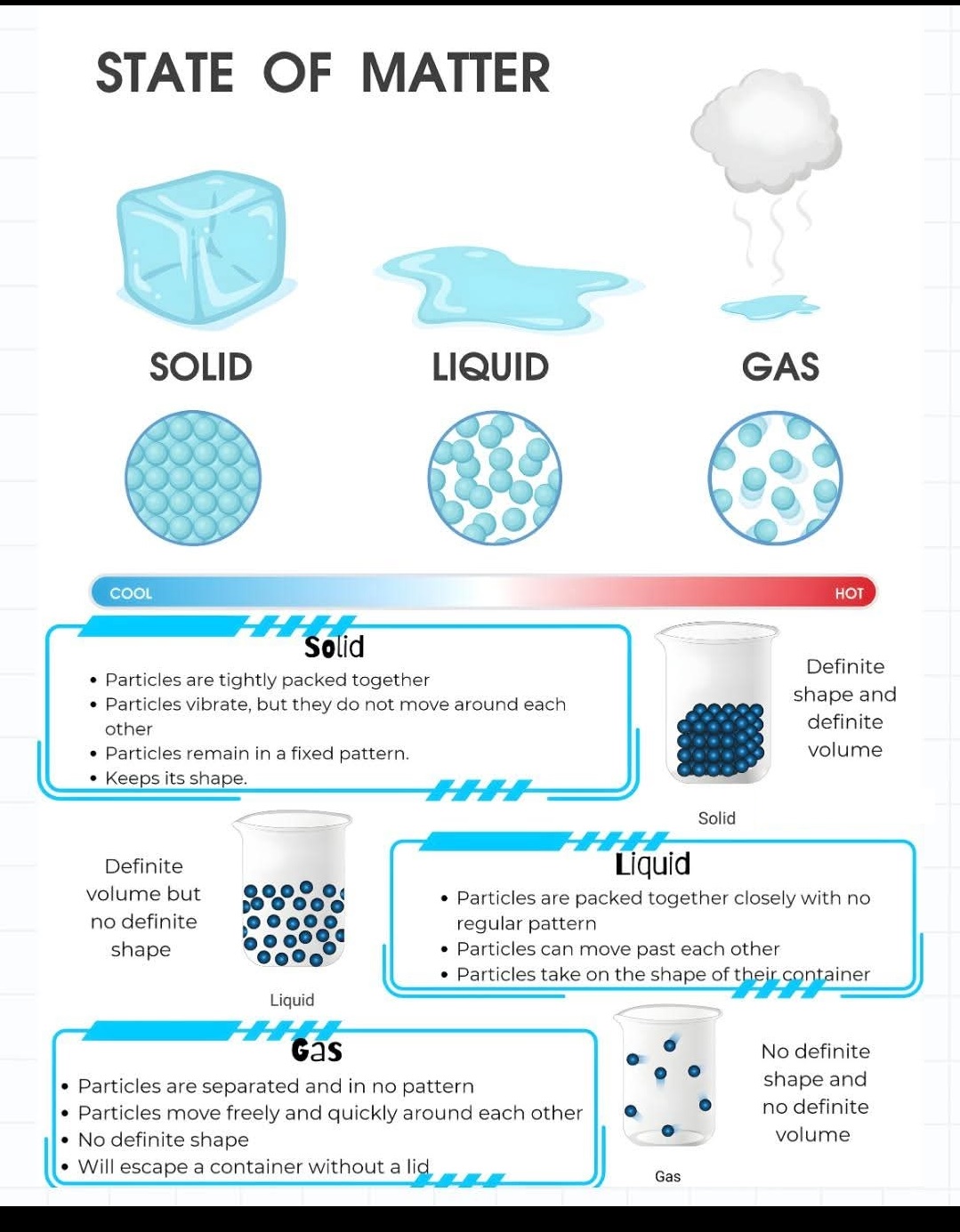
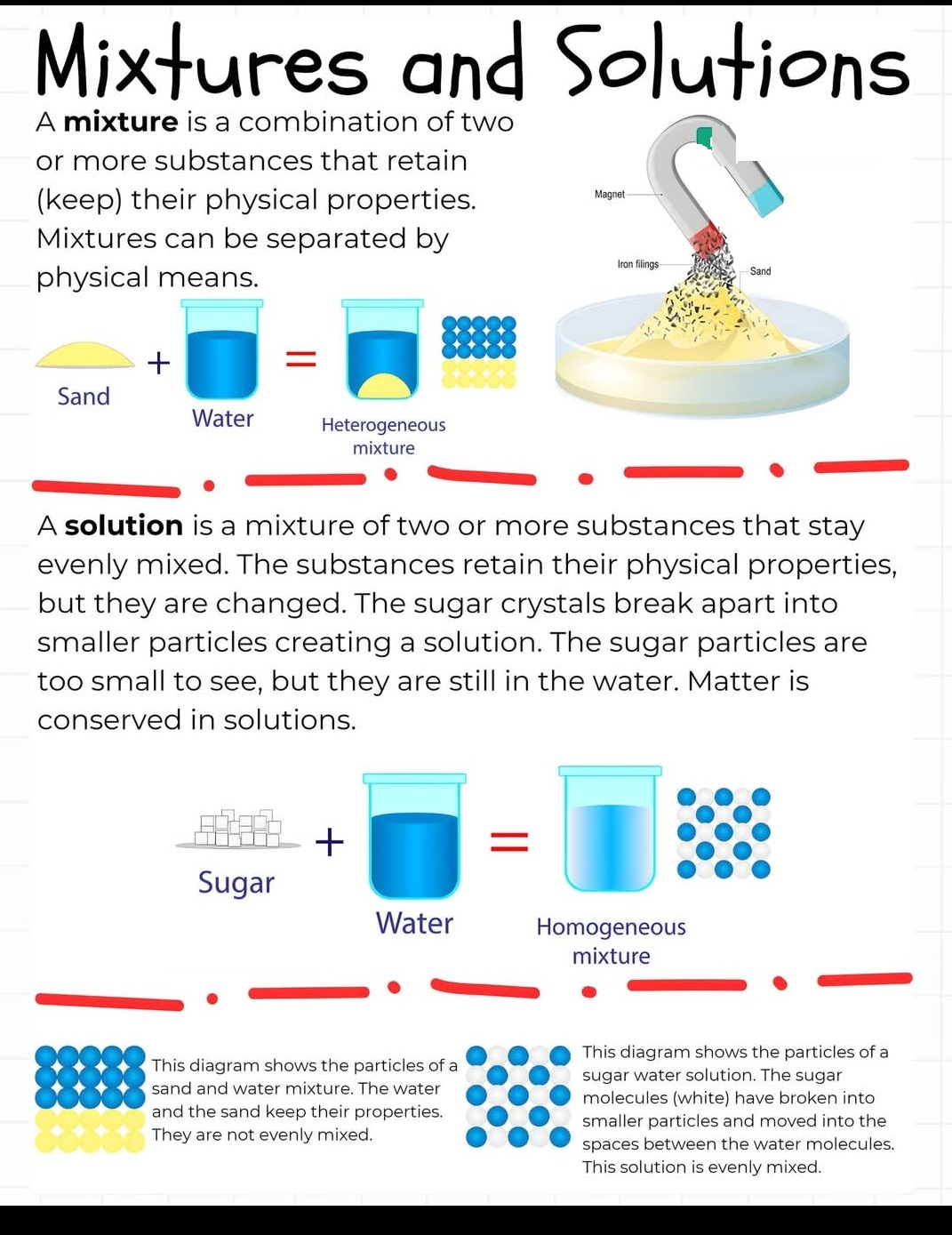
Mixtures and Solutions