Skip to the content
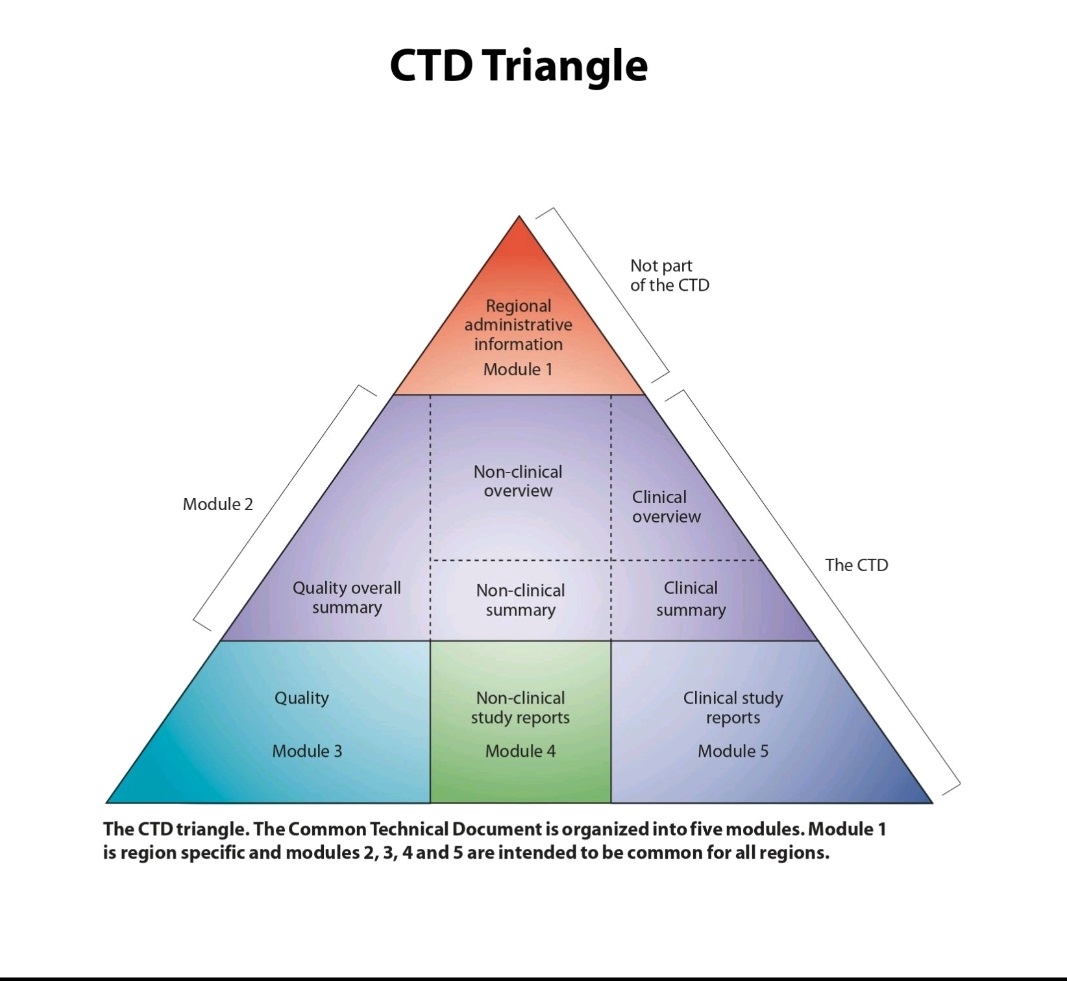
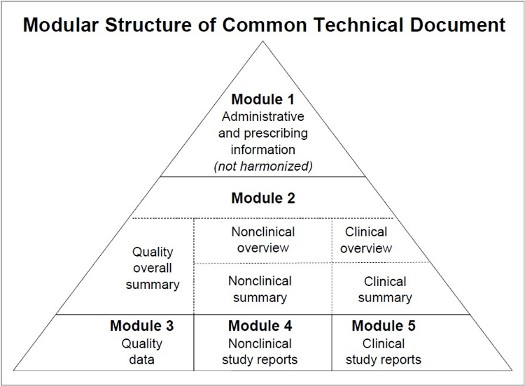
The Five eCTD Modules
Module 1: Administrative Information
- Contains information that is specific to the region where the application is being submitted.
- Includes application forms, cover letters, and other administrative documents
Module 2: Common Technical Document Summaries
-
- Provides summaries and overviews of the technical data in Modules 3, 4, and 5.
- Includes an introduction, a Quality Overall Summary (QOS), and overviews of non-clinical and clinical studie
Module 3: Quality
- Contains detailed information on the quality of the drug substance and product.
- Includes data related to Chemistry, Manufacturing, and Controls (CMC).
Module 4: Non-clinical Study Reports
- Presents the results of non-clinical (pharmacological, toxicological, and pharmacokinetic) studies.
- Typically includes the full study reports
Module 5: Clinical Study Reports
- Contains all the clinical study reports submitted for the product.
- Also includes a tabular listing of all clinical studies.
error: Content is protected !!


