ALCOA
Regulatory Definitions of Data Integrity
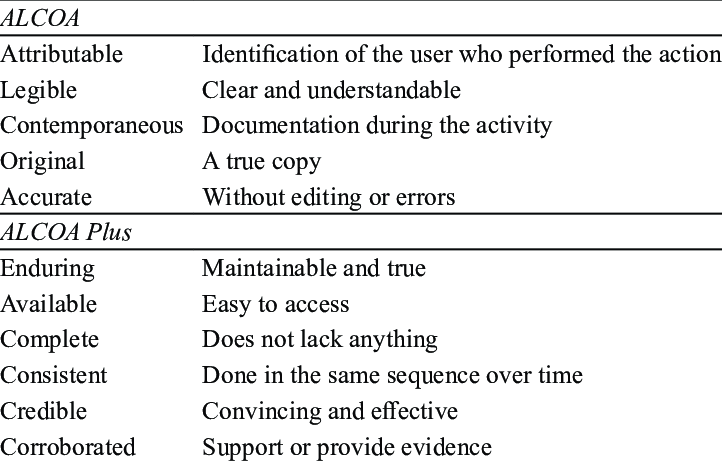
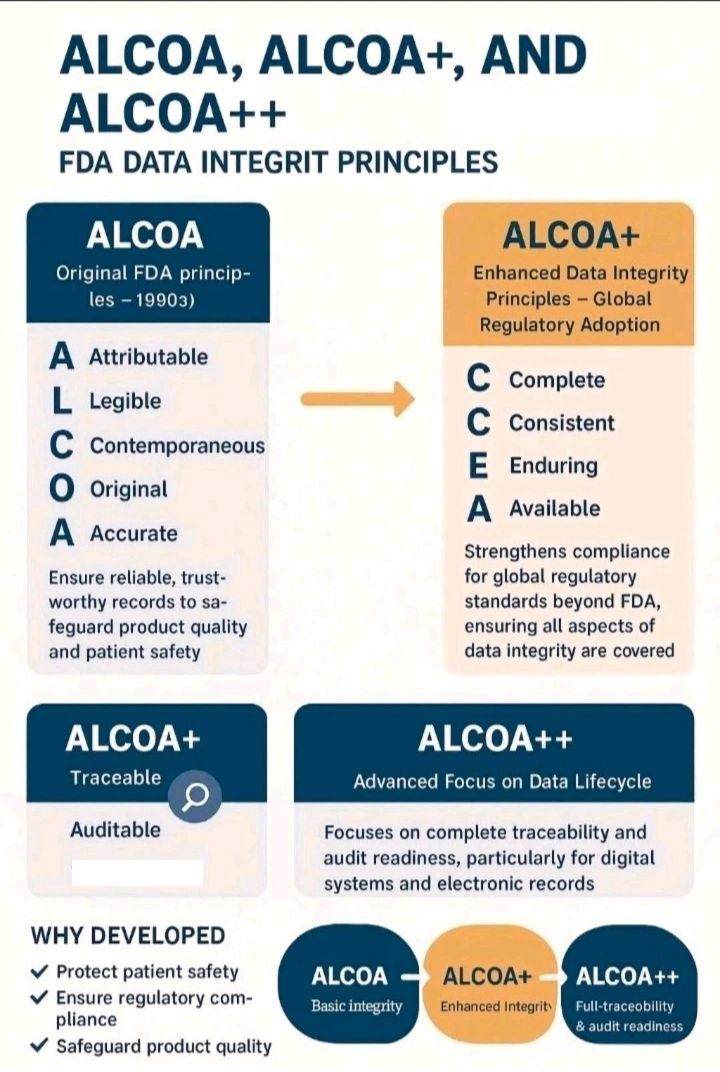
USFDA: “Data integrity refers to the completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded original, and accurate (ALCOA)”.
MHRA: “The extent to which all data are complete, consistent, and accurate throughout the data lifecycle.”
WHO: “Data integrity is the degree to which a collection of data is complete, consistent and accurate throughout the data lifecycle. The collected data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate”.
PICS: “Data Integrity is defined as the extent to which all data are complete, consistent, and accurate, throughout the data lifecycle”.