Title: Determination of Disintegration Test (DT) for Tablets and Capsules
1. Objective: Determination of disintegration test for Tablets and Capsules.
2. Principle: This test determines whether dosage forms such as tablets, capsules, disintegrate within a prescribed time when placed in a liquid medium under the prescribed experimental conditions.
3. Procedure:
3.1 Disintegration Test: DT
For the purpose of this test, disintegration does not imply complete solution of the dosage unit or even of its active constituent. Disintegration is defined as that state in which no residue of the unit under test remains on the screen of the apparatus or, if a residue remains, it consists of fragments of disintegrated parts of tablets component parts such as insoluble coating of the tablets or of capsule shells, or of any melted fatty substance from the pessary or suppository or is a soft mass with no palpable core. If discs have been used with capsules, any residue remaining on the lower surfaces of the discs consists only of fragments of shells.
For Tablets and Capsules
Apparatus
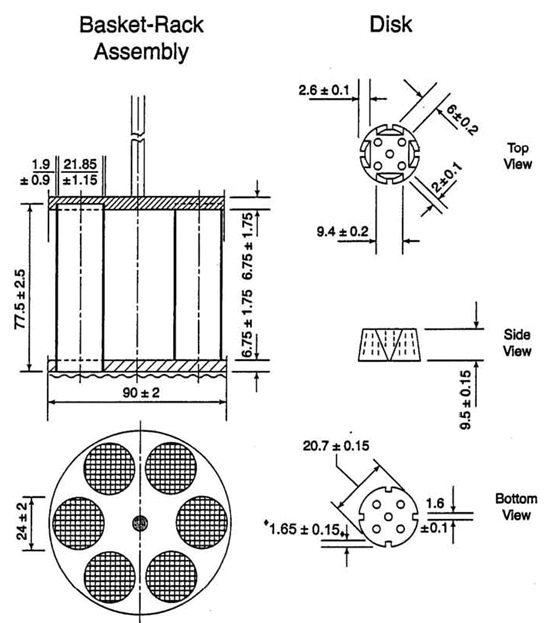
Figure 1. Diagram for disintegration apparatus A (dimensions are expressed in millimetres).
The apparatus consists of a basket-rack assembly, a 1-litre beaker, a thermostatic arrangement for heating the fluid and a mechanical device for raising and lowering the basket in the immersion fluid at a constant frequency rate.
Basket-rack assembly. The basket-rack assembly is rigid and supports six cylindrical glass tubes, 77.5 ± 2.5 mm long, 21.5 mm in internal diameter and with a wall thickness of about 2 mm (Fig.). The tubes are held vertically by two superimposed transparent plastic plates, 90 ± 2mm in diameter and 6.75 ± 1.75 mm thick perforated by six holes having the same diameter as the tubes. The holes are equidistant from the centre of the plate and are equally spaced from one another. Attached to the underside of the lower plate is a woven stainless steel wire cloth with a plain square weave with 2.0 ± 0.2 mm mesh apertures and with a wire diameter of 0.615 ± 0.045 mm. The upper plate is covered with a stainless steel disc perforated by six holes, each about 24 ± 2 mm in diameter, which fits over the tubes and holds them between the plastic plates. The holes coincide with those of the upper plastic plate and the upper open ends of the glass tubes. A suitable means is provided to suspend the basket-rack assembly from the raising and lowering device using a point on its axis.
The plates are held rigidly in position and 77.5 mm apart by vertical metal rods at the periphery and a metal rod is also fixed to the centre of the upper plate to enable the assembly to be attached to the device for raising and lowering it smoothly at a constant frequency of between 28 and 32 cycles per minute through a distance of 50 to 60 mm. The time required for the upward stroke is equal to the time required for the downward stroke, and the change in stroke direction should be smooth and not abrupt. There should be no appreciable horizontal motion or movement of the axis from the vertical.
The design of the basket-rack assembly may be somewhat different provided specifications for the glass tubes and the screen mesh size are unchanged.
Discs. A cylindrical disc for each tube, each 20.7 ± 0.15 mm thick in diameter and 9.5 ± 0.15 mm thick, made of transparent plastic with a relative density of 1.18 to 1.20, and pierced with five holes, each 2 mm in diameter, one in the centre and the other four spaced equally on a circle of radius 6 mm from the centre of the disc. Four equally-spaced grooves are cut in the lateral surface of the disc in such a way that at the upper surface of the disc they are 9.5 mm wide and 2.55 mm deep and at the lower surface 1.6 mm square.
Medium
The assembly is suspended in the liquid medium in a suitable vessel, preferably a 1-litre beaker. The volume of liquid is such that the wire mesh at its highest point is at least 25 mm below the surface of the liquid, and at its lower point is at least 25 mm above the bottom of the beaker. At no time should the top of the basket-rack assembly become submerged. There is a thermostatic arrangement for heating the liquid and maintaining the temperature at 37°C ± 2°C.
Method
Unless otherwise stated in the individual monograph, introduce one tablet or capsule into each tube and, if directed in the appropriate general monograph, add a disc to each tube. Suspend the assembly in the beaker containing the specified liquid and operate the apparatus for the specified time. Remove the assembly from the liquid. The tablets or capsules pass the test if all of them have disintegrated.
If 1 or 2 tablets or capsules fail to disintegrate, repeat the test on 12 additional tablets or capsules; not less than 16 of the total of 18 tablets or capsules tested disintegrate. If the tablets or capsules adhere to the disc and the preparation under examination fails to comply, repeat the test omitting the disc. The preparation complies with the test if all the tablets or capsules in the repeat test disintegrate.
For enteric-coated tablets
Apparatus
Use the apparatus for tablets and capsules described above.
Method
Put one tablet into each tube, suspend the assembly in the beaker containing 0.1 M hydrochloric acid and operate without the discs for 2 hours, unless otherwise stated in the individual monograph. Remove the assembly from the liquid.
No tablet shows signs of cracks that would allow the escape of the contents or disintegration, apart from fragments of coating.
Replace the liquid in the beaker with mixed phosphate buffer pH 6.8, add a disc to each tube and operate the apparatus for a further 60 minutes. Remove the assembly from the liquid. If the tablet fails to comply because of adherence to the disc, repeat the test on a further 6 tablets without the discs. The tablets pass the test if all six have disintegrated.
