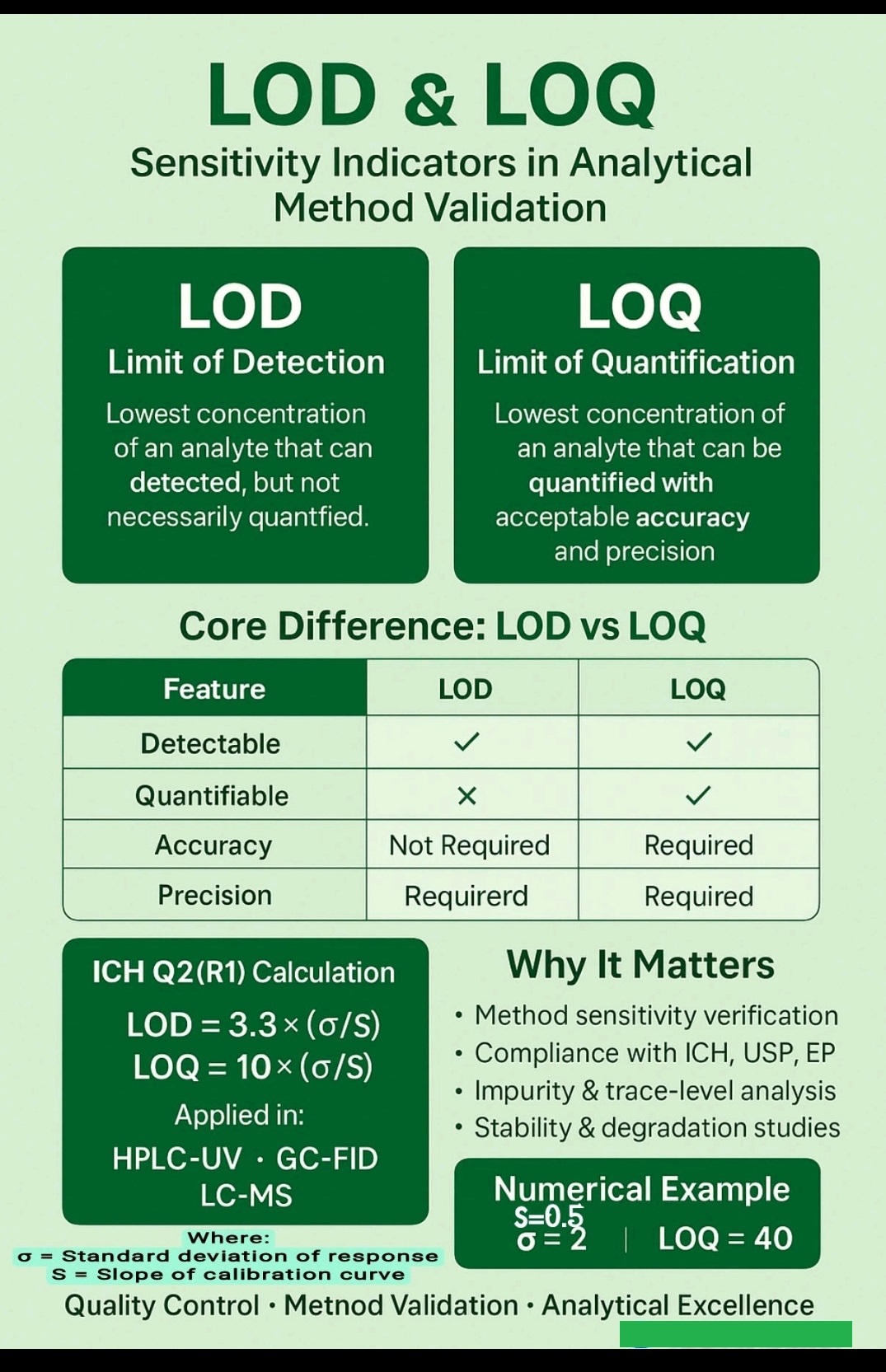
LOD vs LOQ
Method Validation, Limit of Detection, Limit of Quantification, Signal to Noise ratio.

Pharma Definition/Abbreviation, Pharma Beginners
Method Validation, Limit of Detection, Limit of Quantification, Signal to Noise ratio.




ISO 9001, ISO 14001, ISO 45001, ISO 22000, ISO 27001, ISO 50001, ISO 31000, ISO 13485, ISO 17025, ISO 21001, ISO 22301.

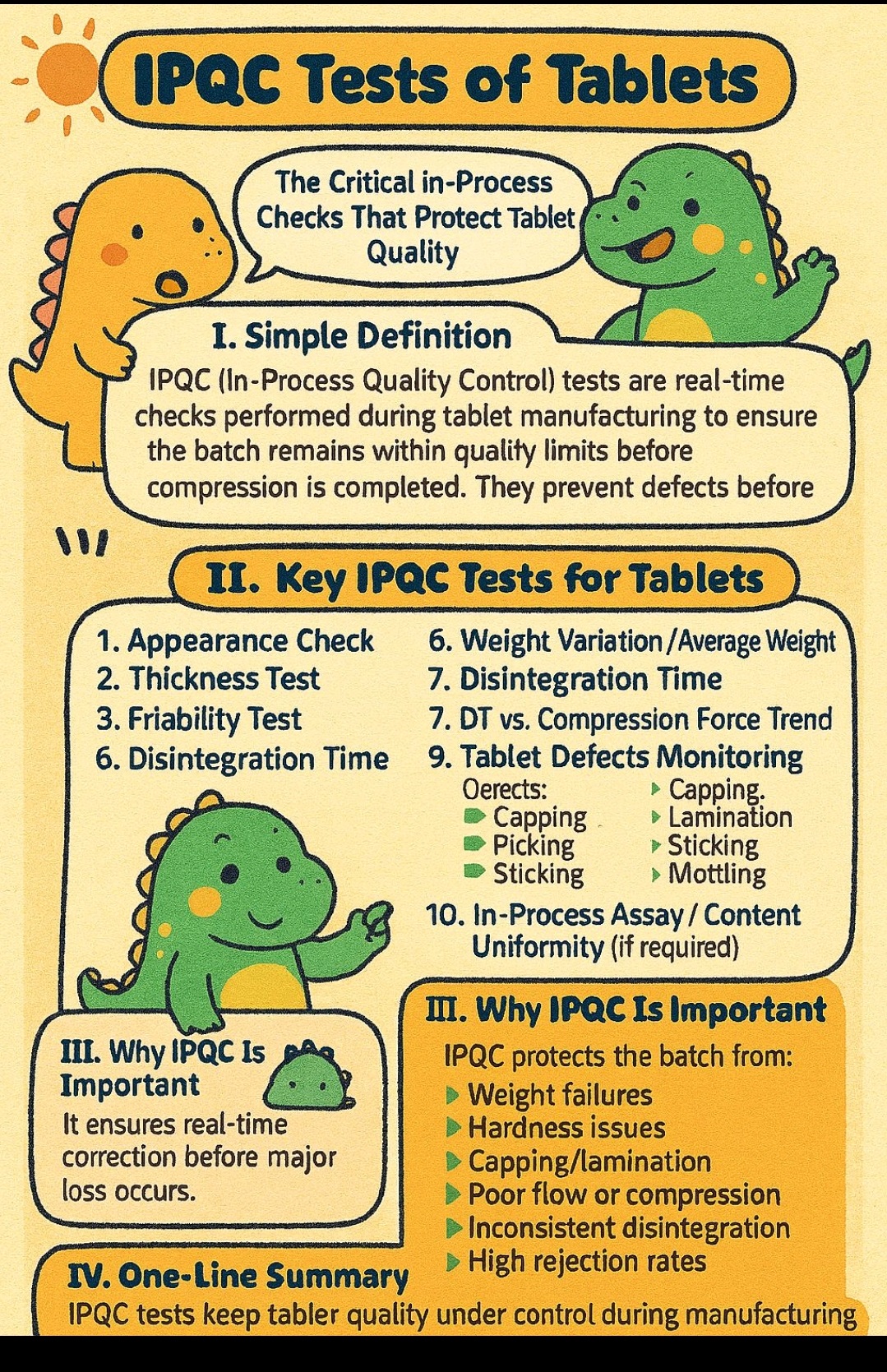
IPQC- Thickness, hardness, Friability, DT, weight variation, Defects, Assay, content uniformity.
Fire safety
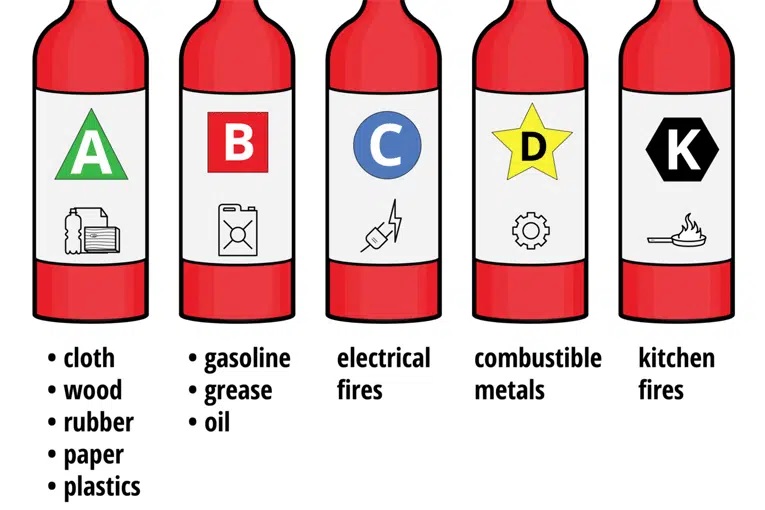
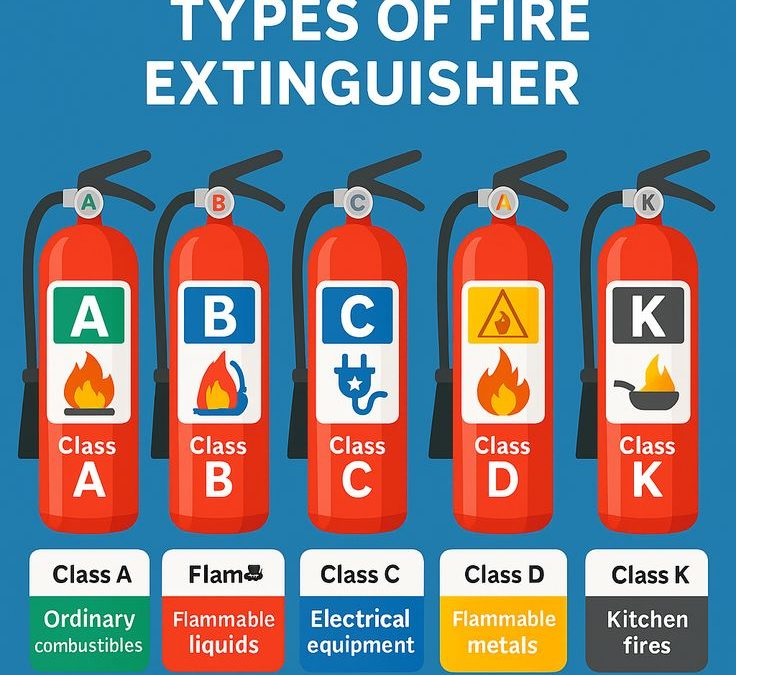
Types of Fire
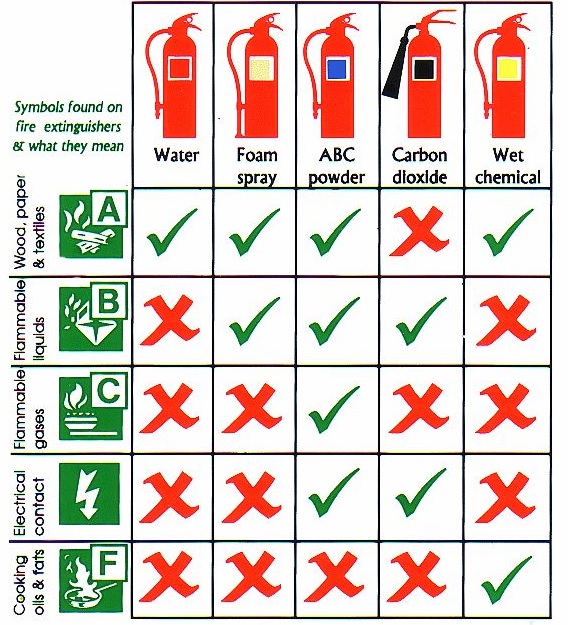
Class A: fire extinguisher – water, water mist, foam, dry powder, wet chemical.
Class B: fire extinguisher – water mist, foam, dry powder, CO2, some wet chemical.
Class C: fire extinguisher – water mist, dry powder.
Class D: fire extinguisher – specialist dry powder.
Class K: fire extinguisher – wet chemical agents.

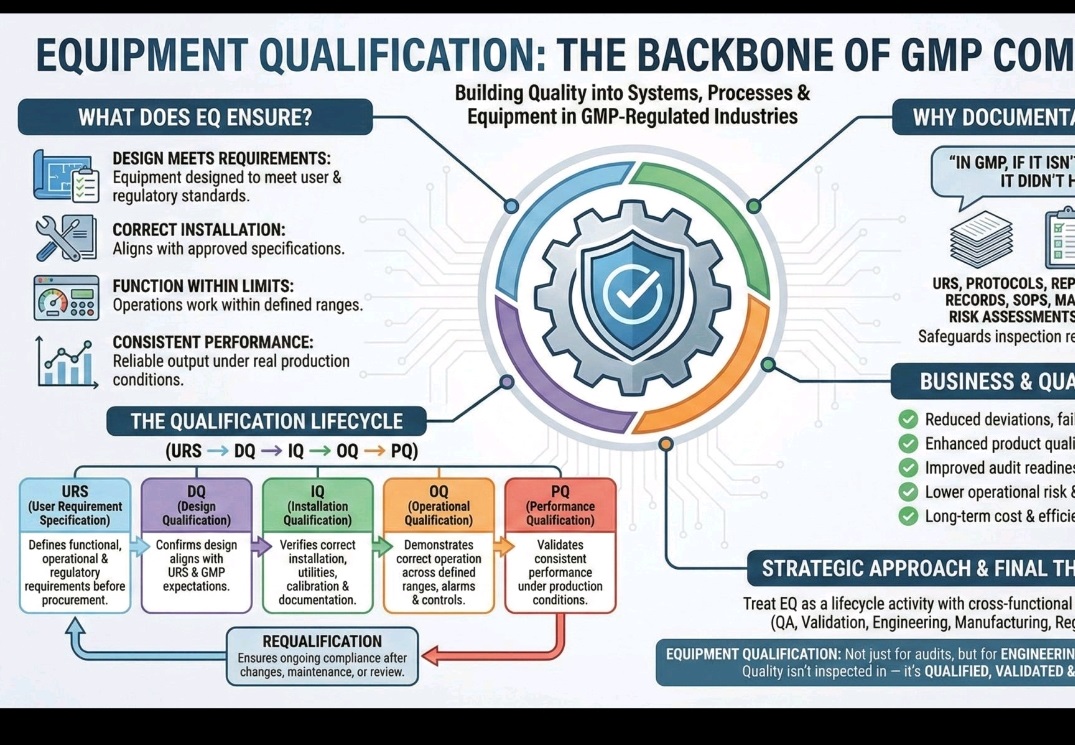
URS/DQ/IQ/OQ/PQ/Qualification life cycle/GMP/Requalification
Cleaning Methods in Pharma
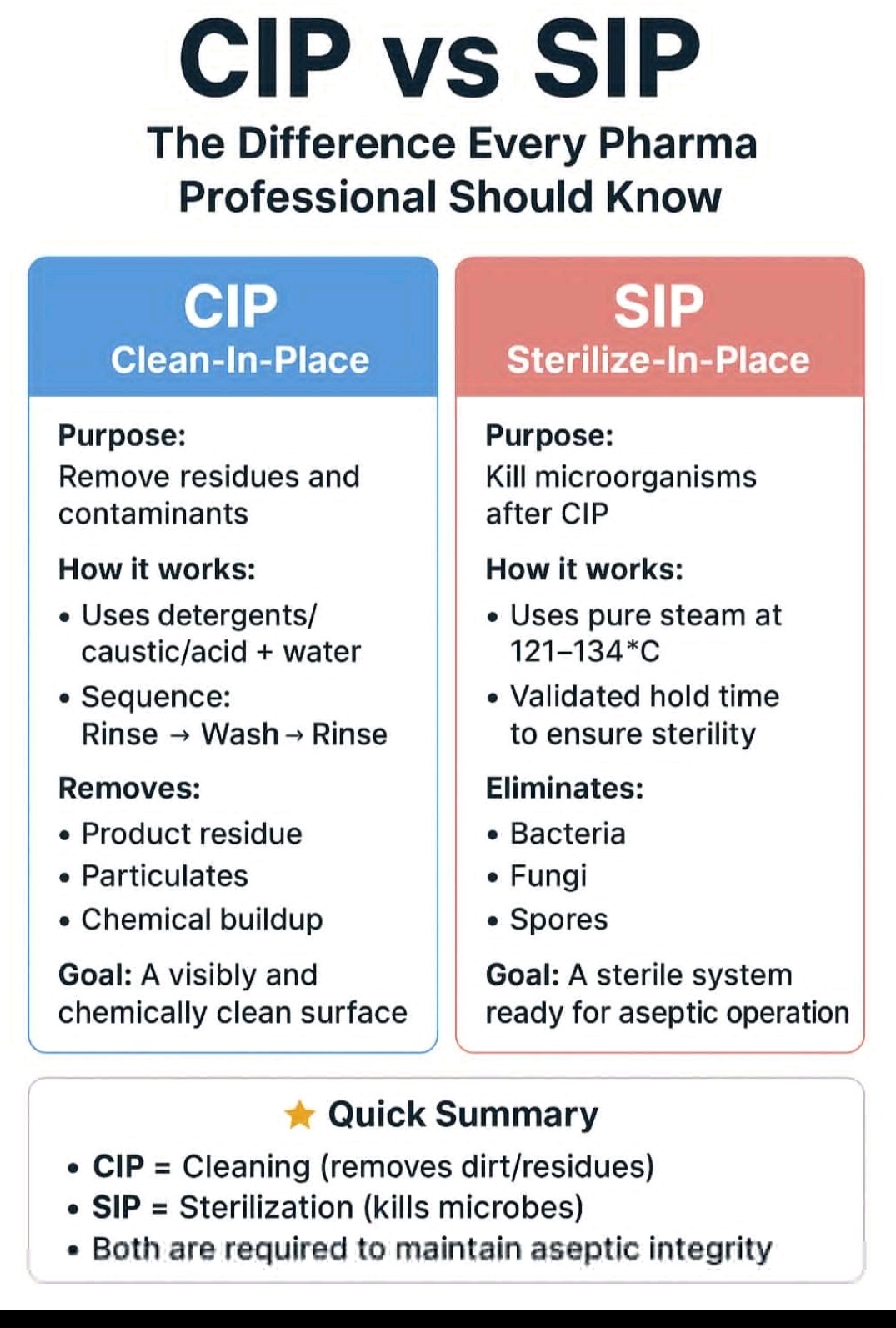
Internal Audit, External Audit,Certification Audit, Process Audit, Product Audit, System Audit,Supplier Audit, Compliance Audit.
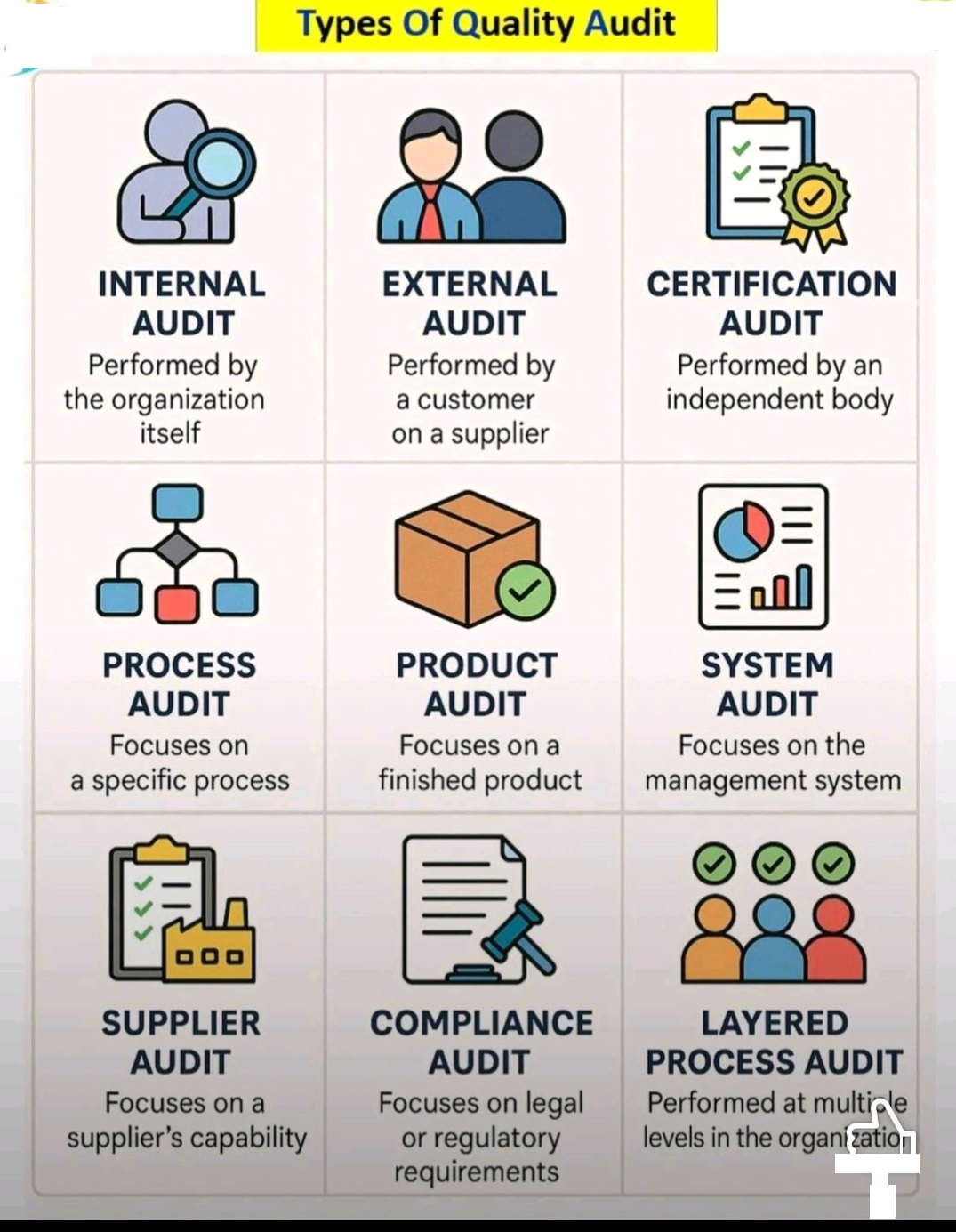


amoxicillin, ampicillin, cephalexin, cefazolin, azithromycin, erythromycin, ciprofloxacin, levofloxacin, doxycycline, minocycline, gentamicin, tobramycin, vancomycin, tetracyclines, macrolides
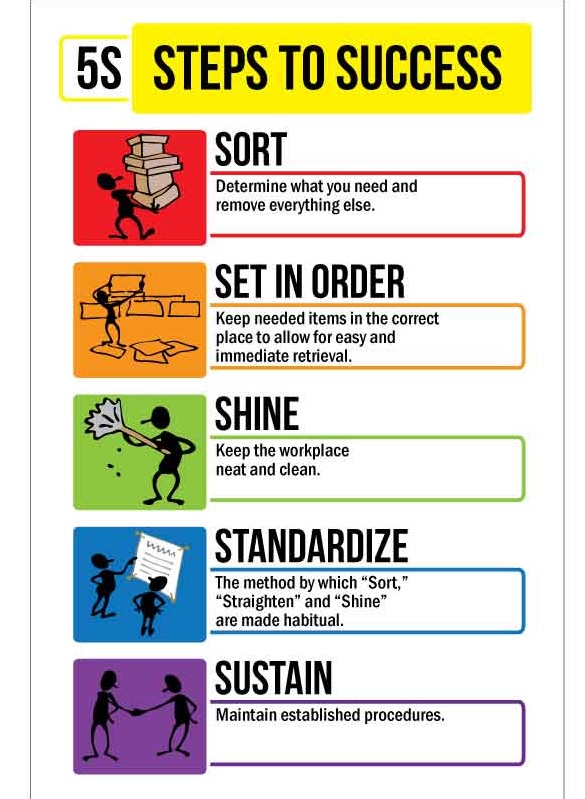
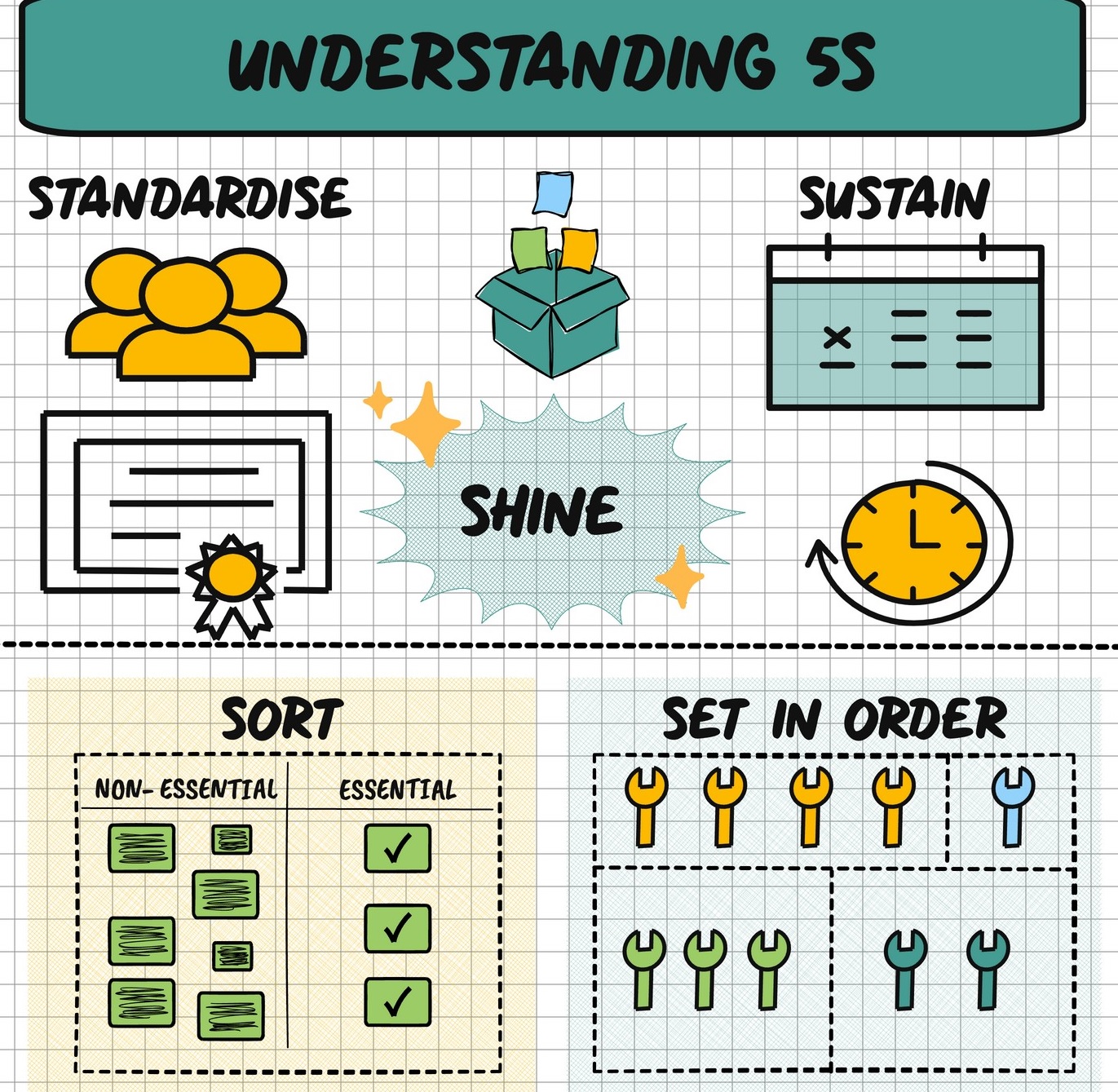
The 5S step is a Lean methodology for workplace organization, focusing on five Japanese words starting with ‘S’
2. Set in Order (Seiton): Arrange necessary items so they are easy to find, use, and return, with a designated place for everything (e.g., “a place for everything, and everything in its place”).
3. Shine (Seiso): Clean the work area thoroughly and regularly, making cleaning part of the routine to prevent issues and maintain safety.
4. Standardize (Seiketsu): Create procedures, checklists, and schedules to ensure the first three Ss (Sort, Set in Order, Shine) are consistently followed.
5. Sustain (Shitsuke): Develop the discipline to maintain the standards over time through audits, training, and management commitment, turning 5S into a habit.