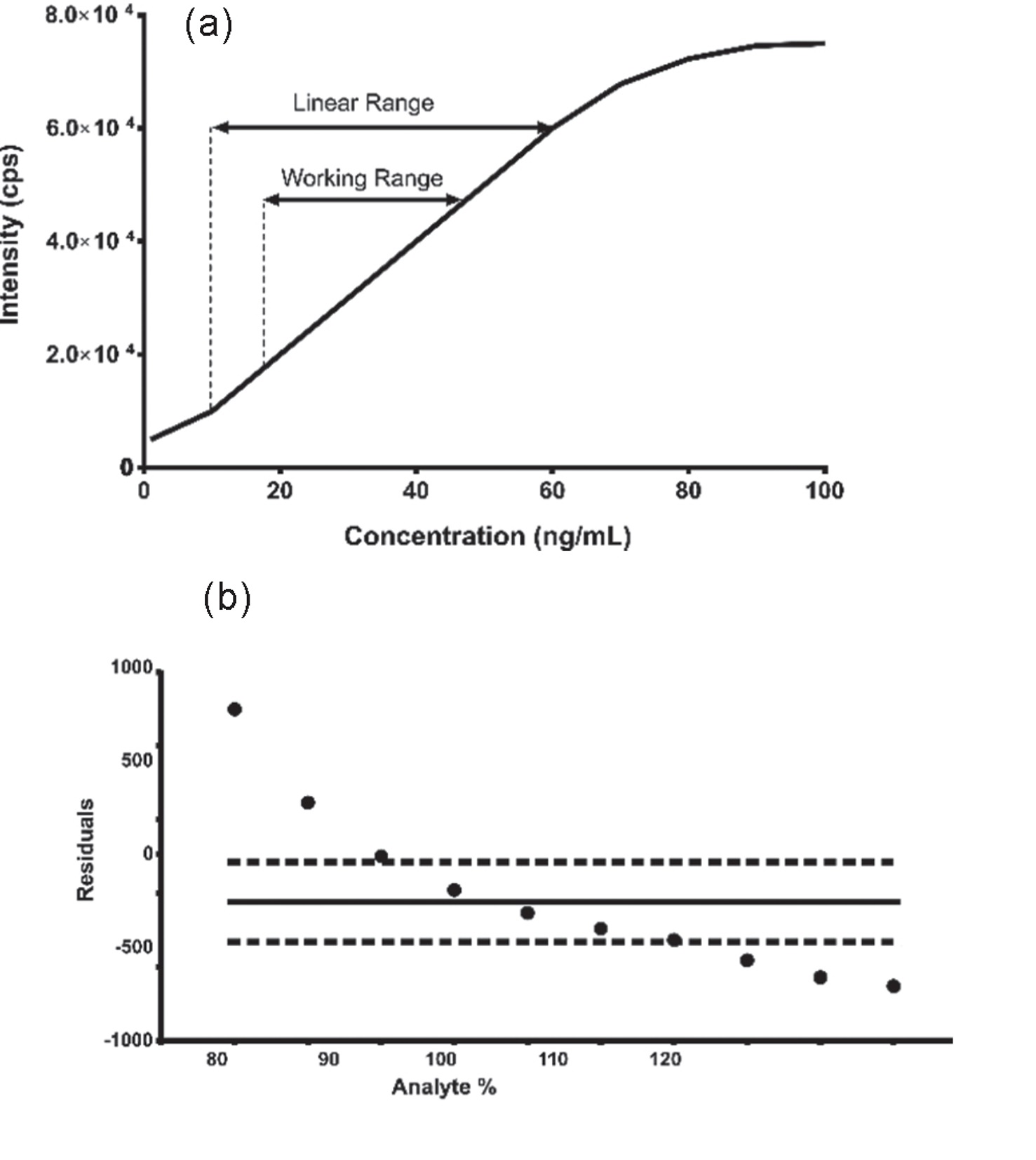
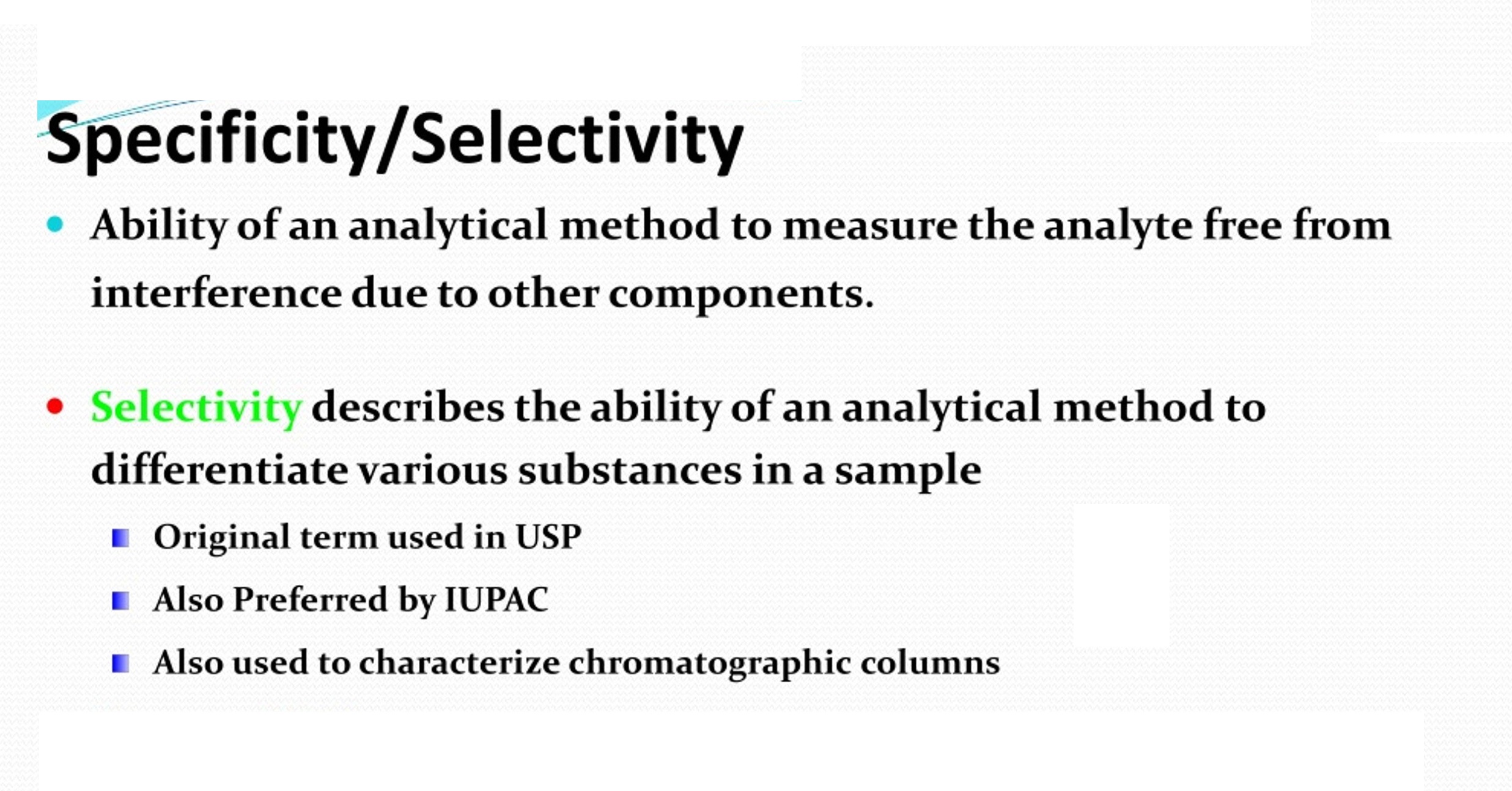
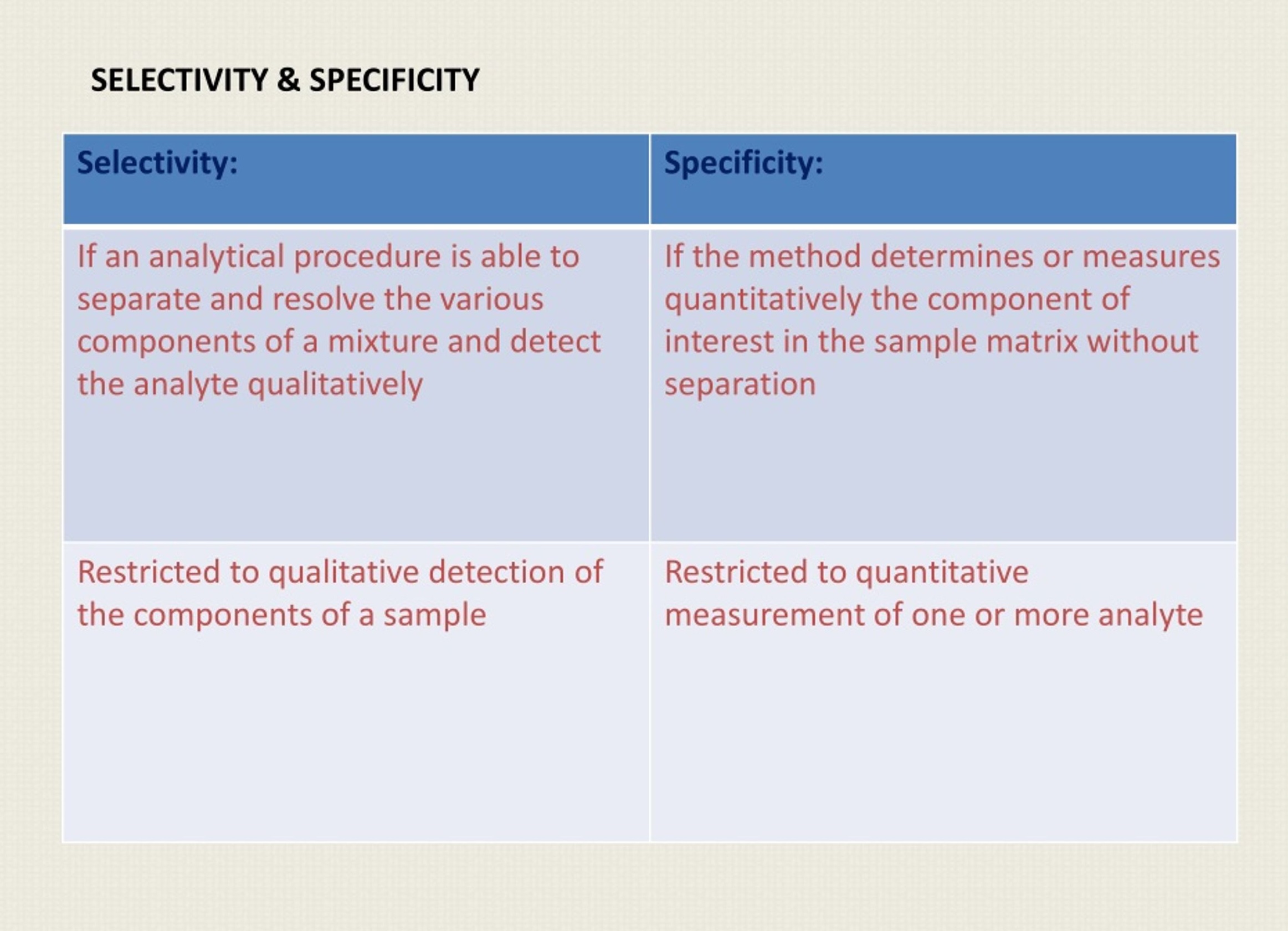
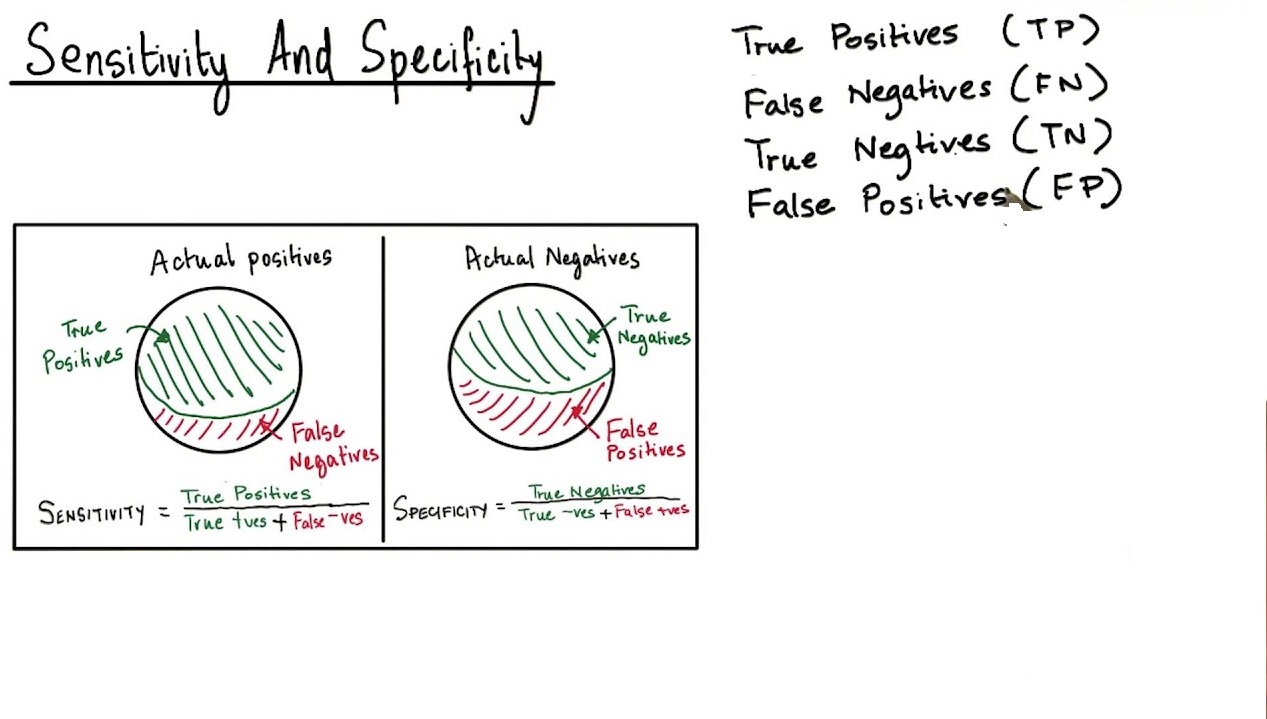
Solution Stability vs Force Degradation
Analytical Method Validation
Solution stability tests how a drug behaves under normal storage (room temp, fridge, light protection) over time, while
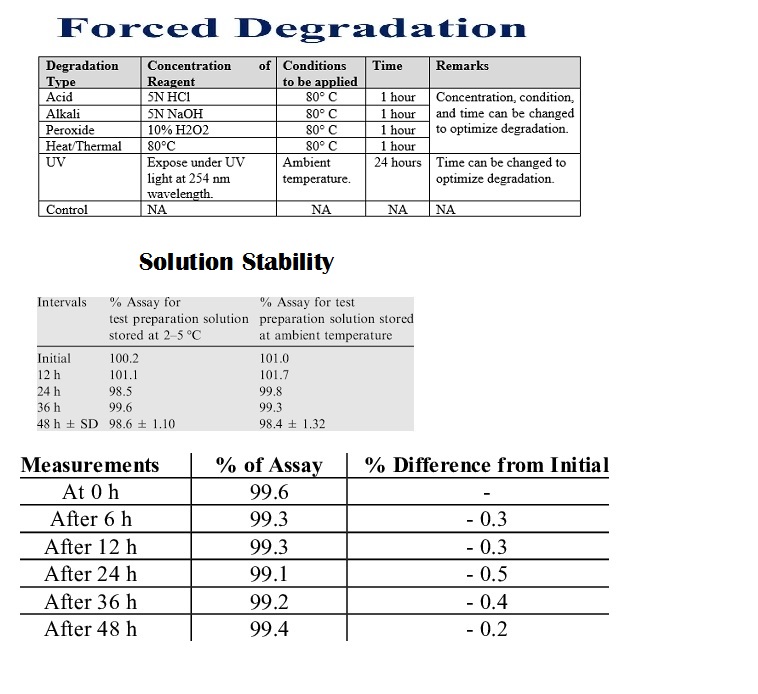
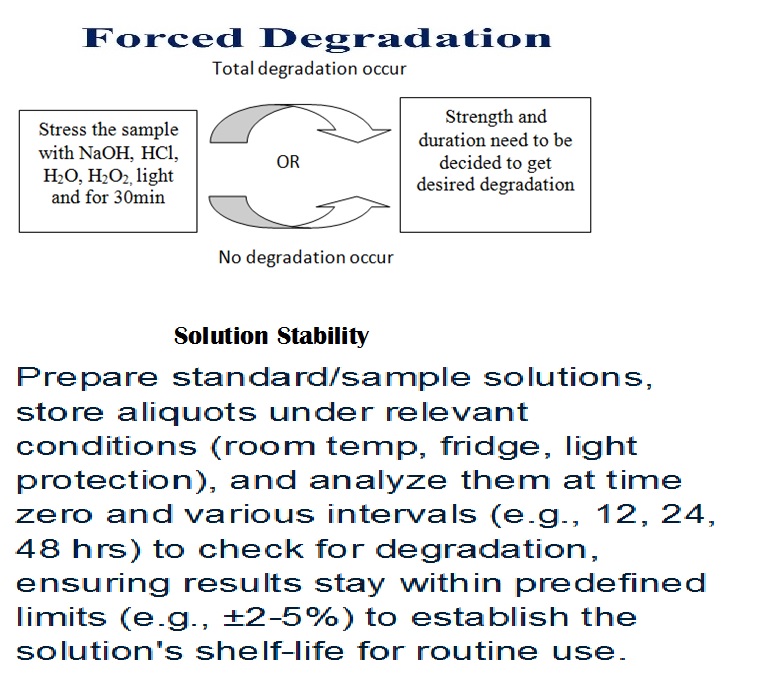
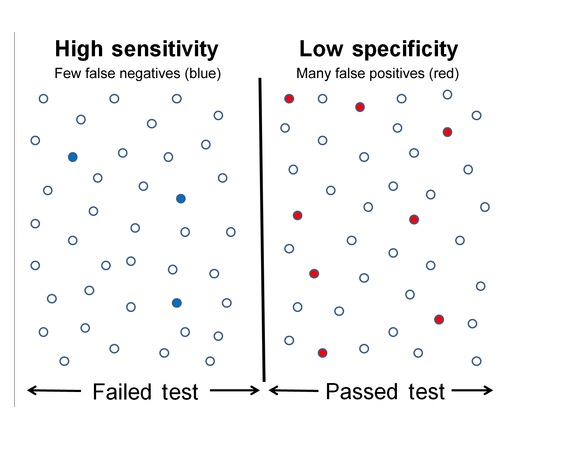
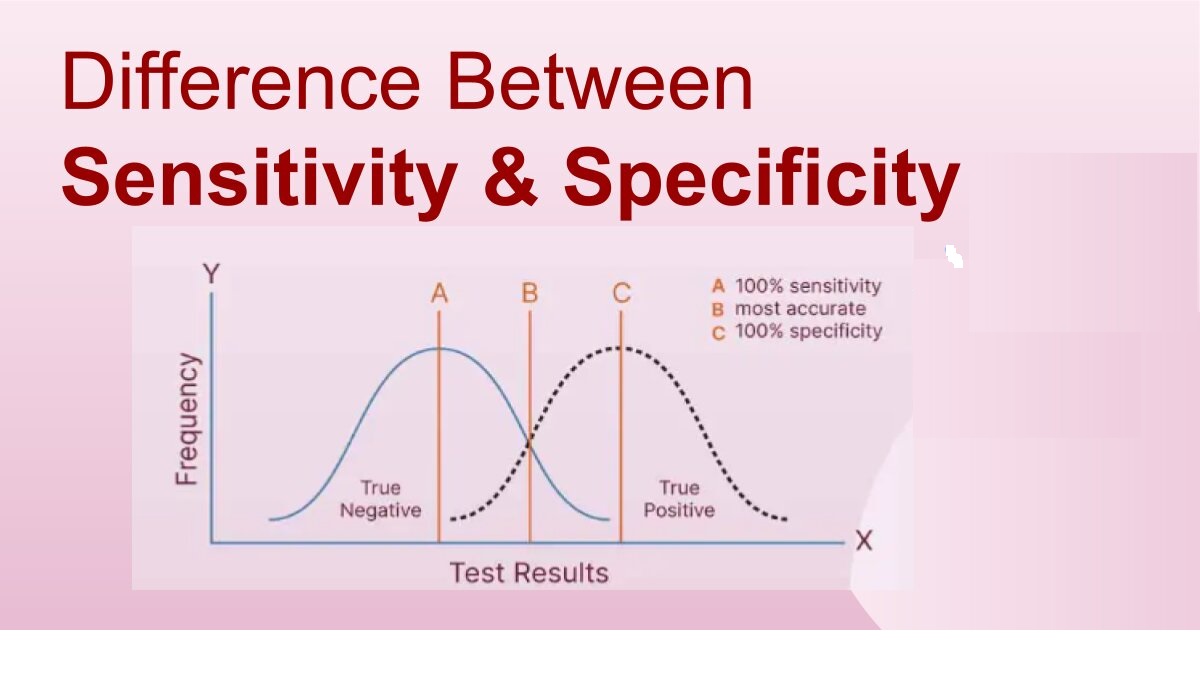
Forced degradation (stress testing) uses extreme conditions (acid/base, heat, oxidation, light) to rapidly identify potential breakdown products, establish degradation pathways, and ensure the analytical method can separate the main drug from these impurities, proving it’s “stability-indicating”
Solution stability acceptance criteria in analytical validation ensure prepared standards and samples remain consistent over time, typically requiring the assay result (e.g., % purity) to stay within ±2% of the initial value, with impurity levels controlled within tighter ranges (e.g., ±0.04% total) for a defined period (e.g., hours to days at room or refrigerated temp).
prepare standard/sample solutions, store aliquots under relevant conditions (room temp, fridge, light protection), and analyze them at time zero and various intervals (e.g., 2,4,6,12, 24, 48 hrs) to check for degradation, ensuring results stay within predefined limits (e.g., ±2-5%) to establish the solution’s shelf-life for routine use.