Polar Molecule vs Non Polar Molecule
Polar solvents have uneven electron distribution, leading to partial charges and a dipole moment, while nonpolar solvents have an even electron distribution and no dipole moment.
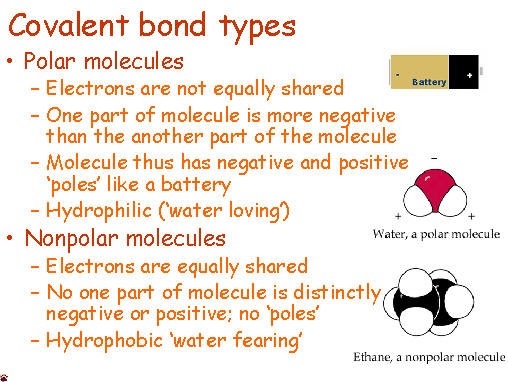
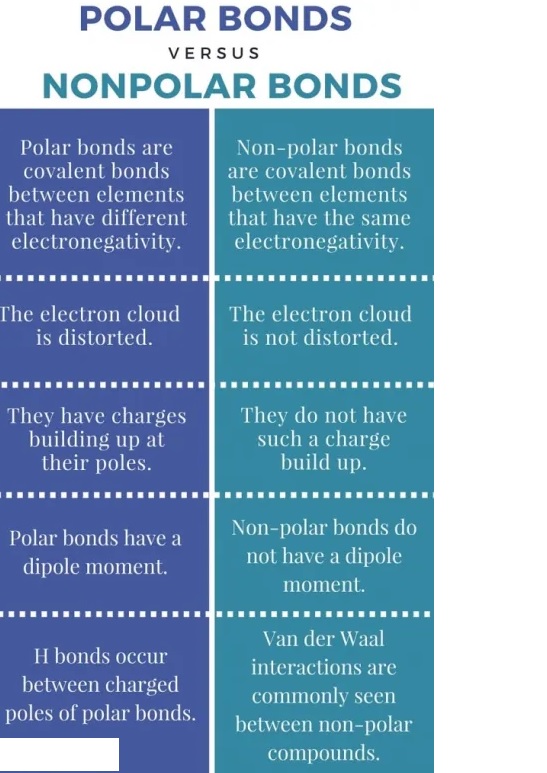
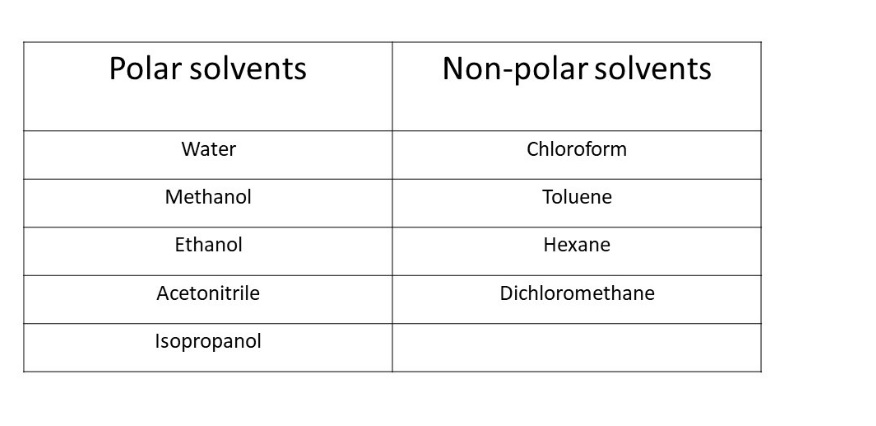
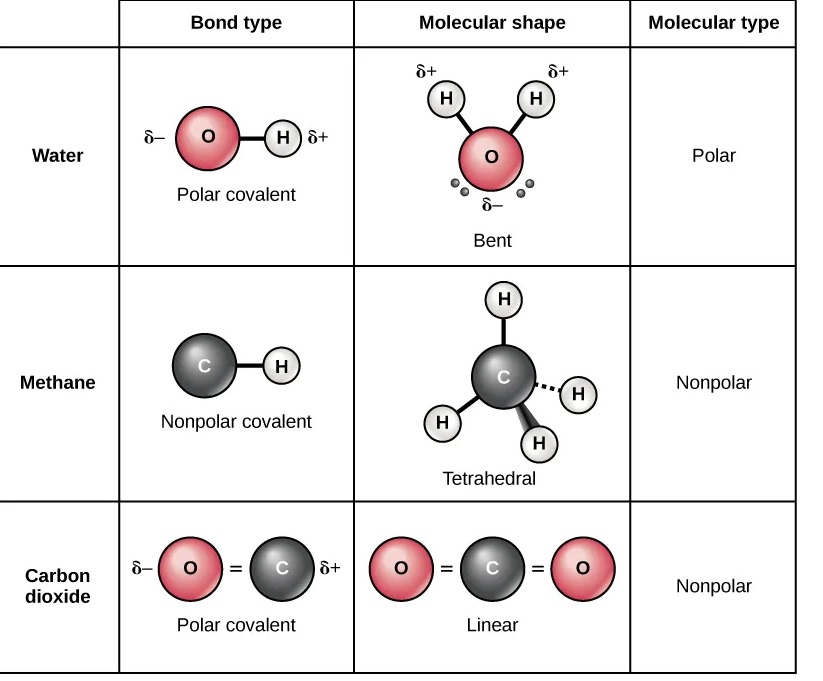
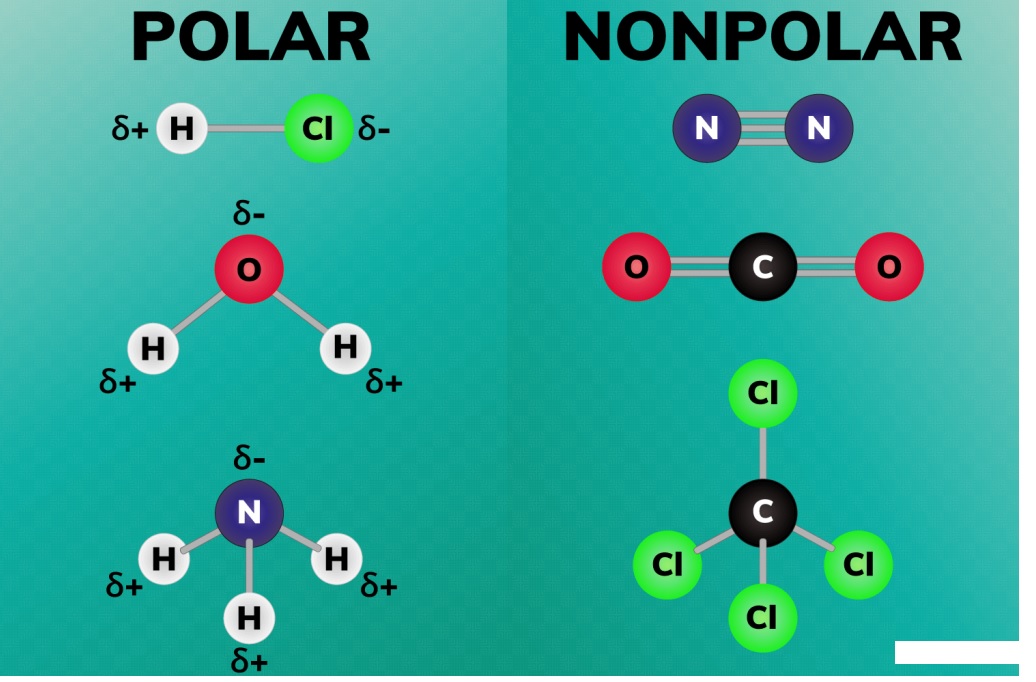
Polar solvents have uneven electron distribution, leading to partial charges and a dipole moment, while nonpolar solvents have an even electron distribution and no dipole moment.




