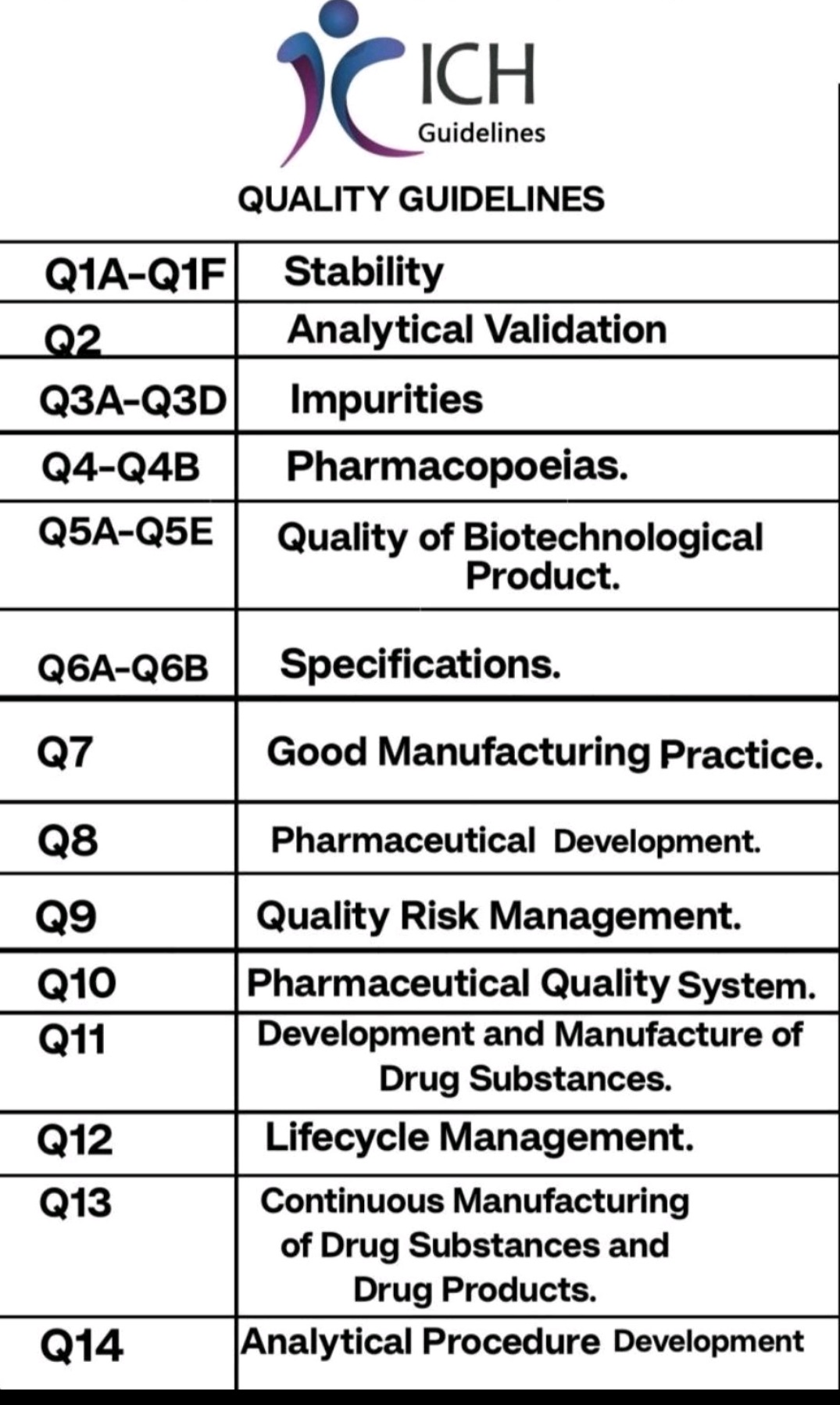
ICH Guideline-Quality


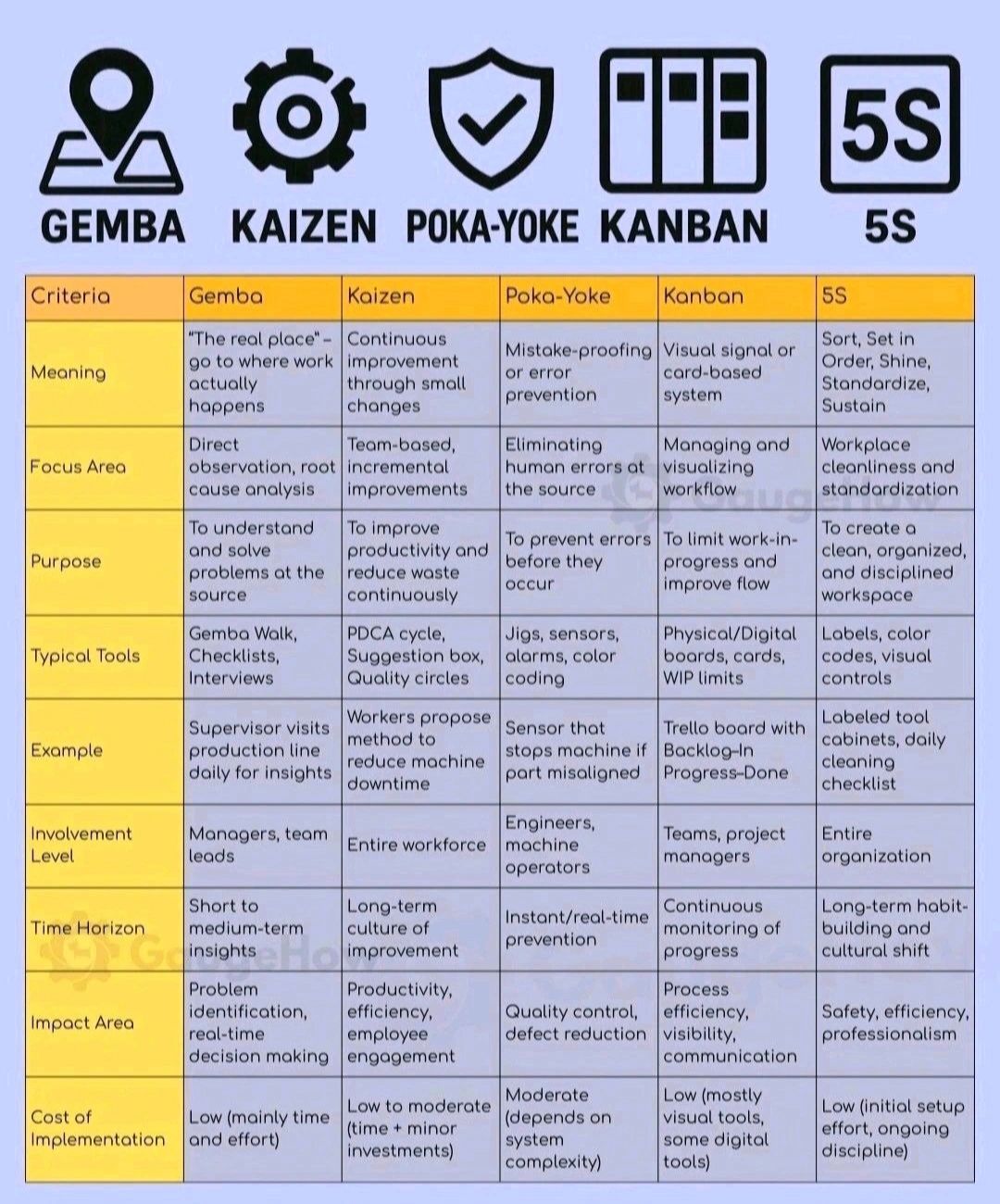
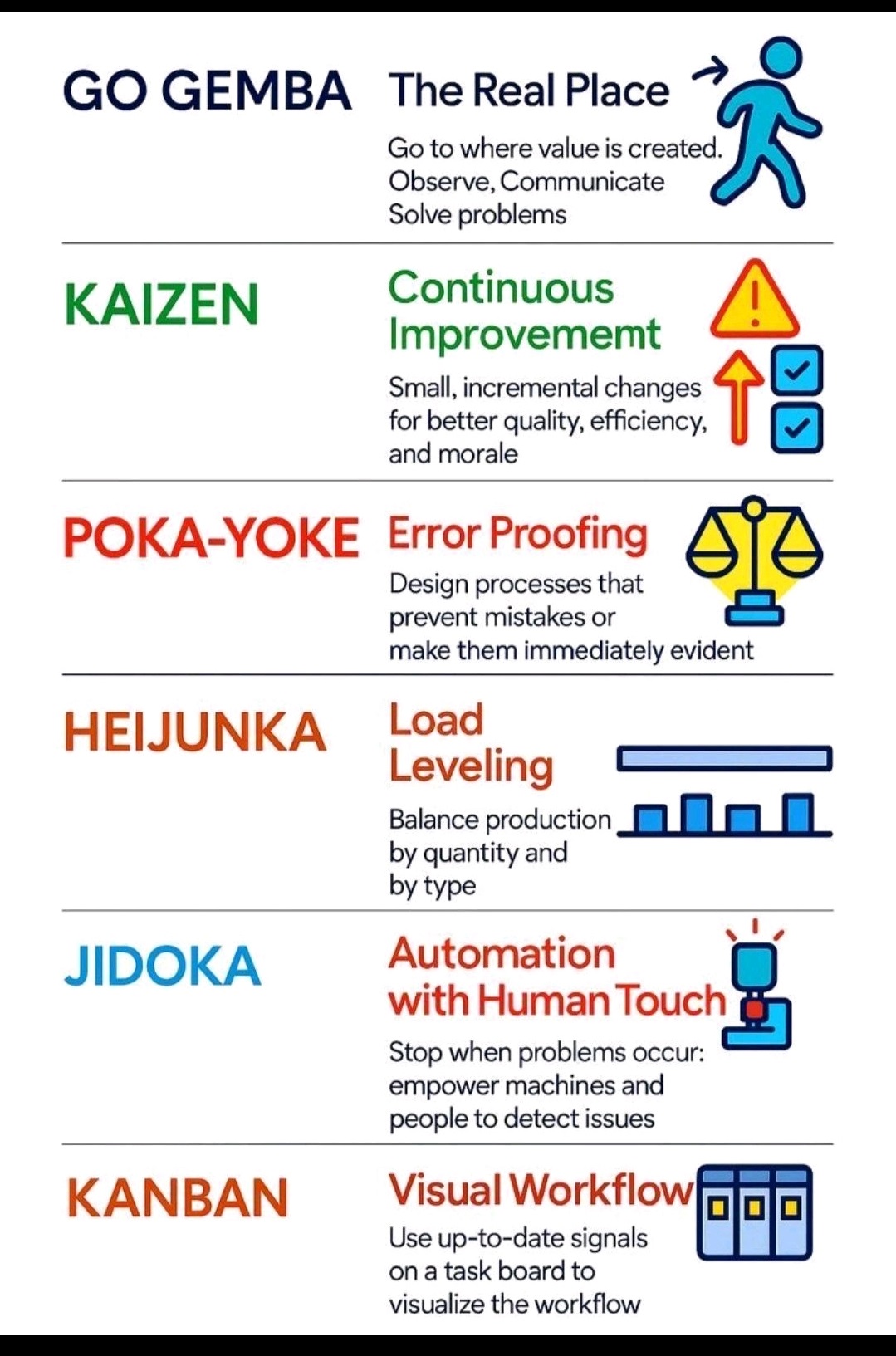
Pharma Definition/Abbreviation, Pharma Beginners



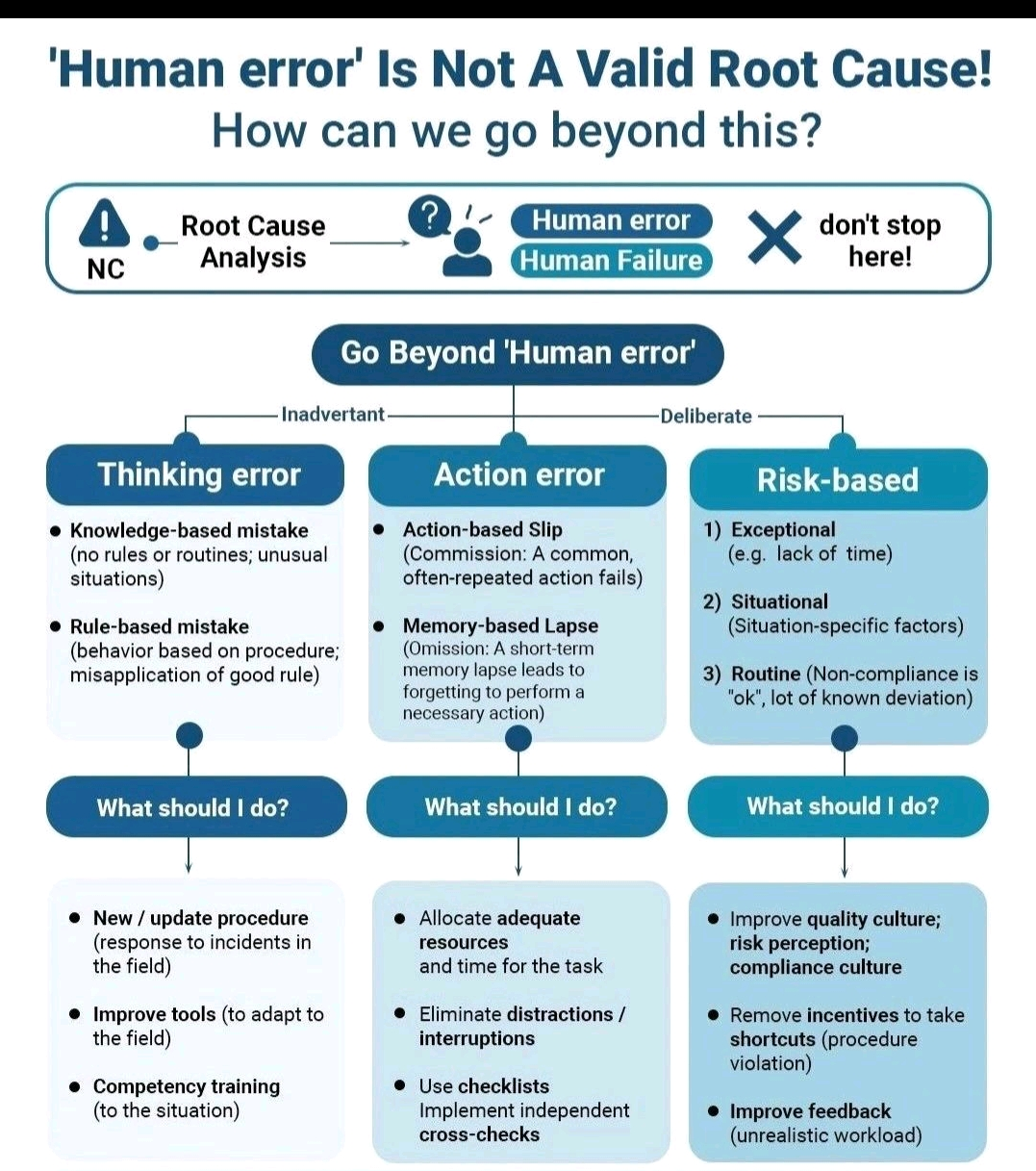
21 CFR refers to Title 21 of the Code of Federal Regulations, which contains the rules and regulations for the Food and Drug Administration (US FDA).
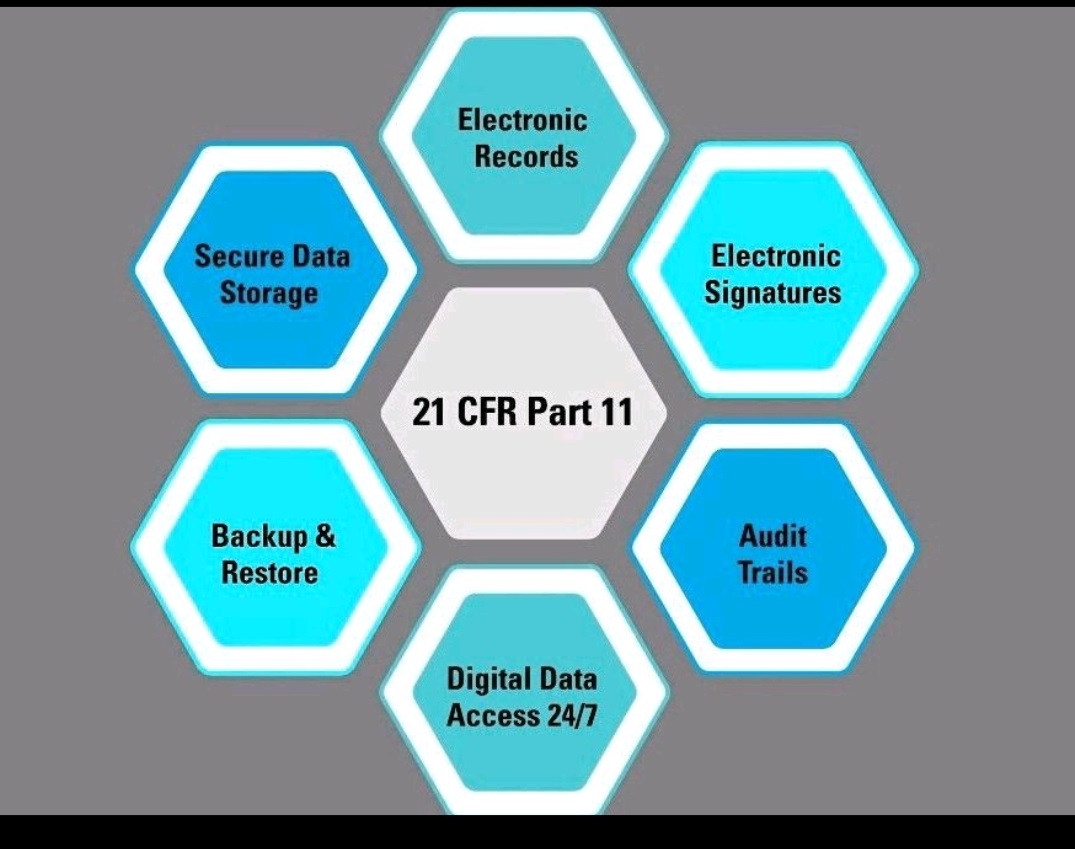
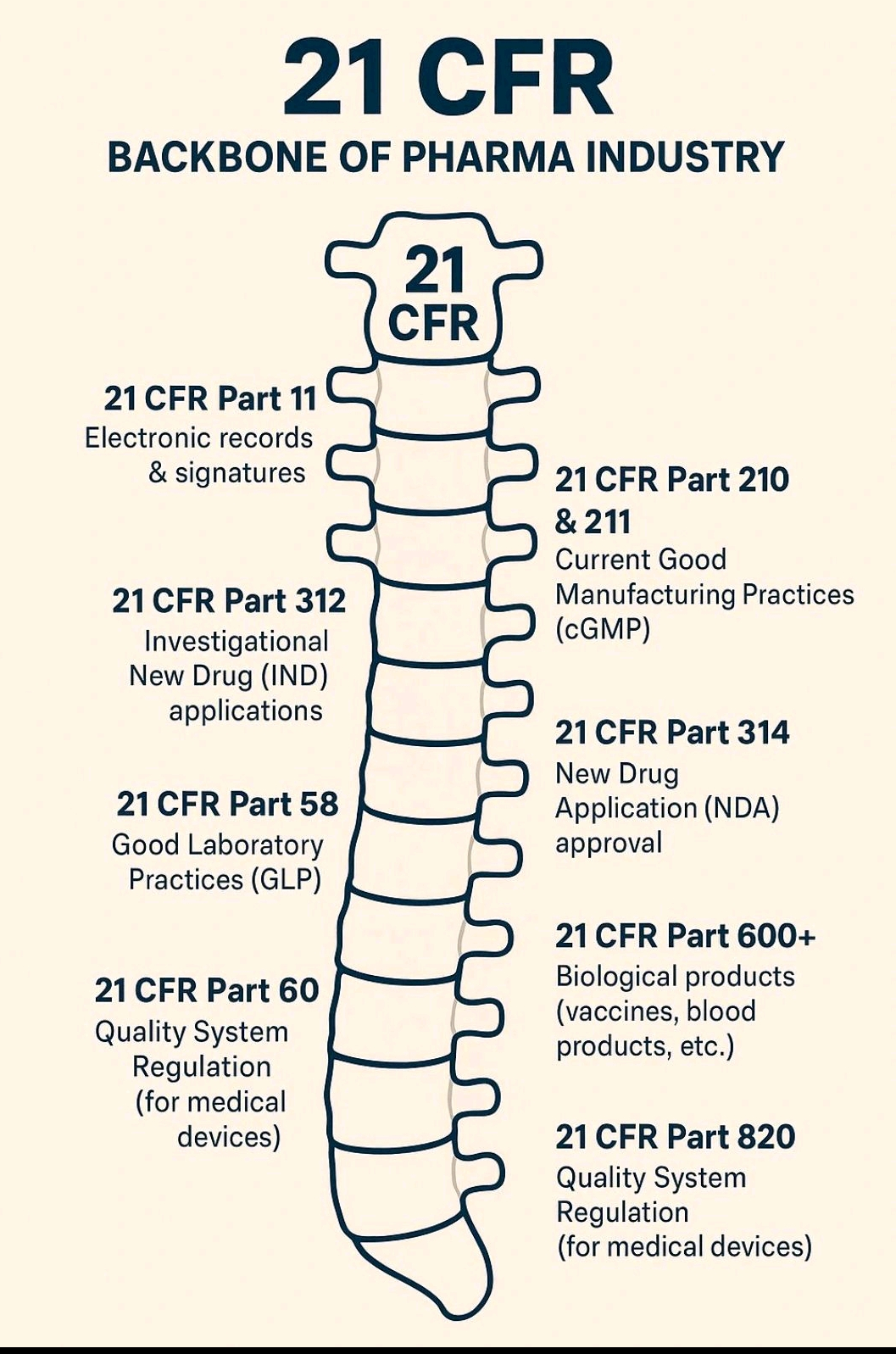
21 CFR part 11 & EU Annex 11

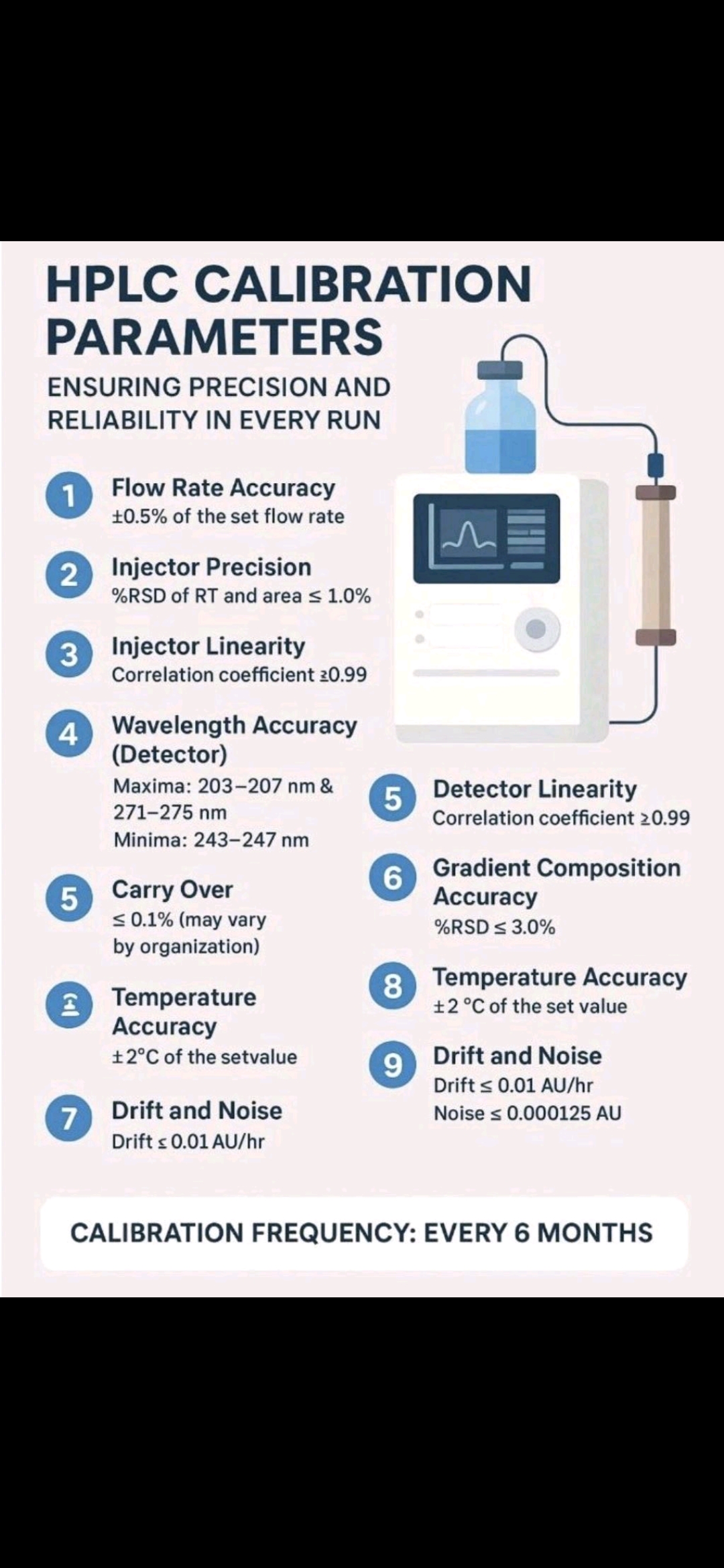
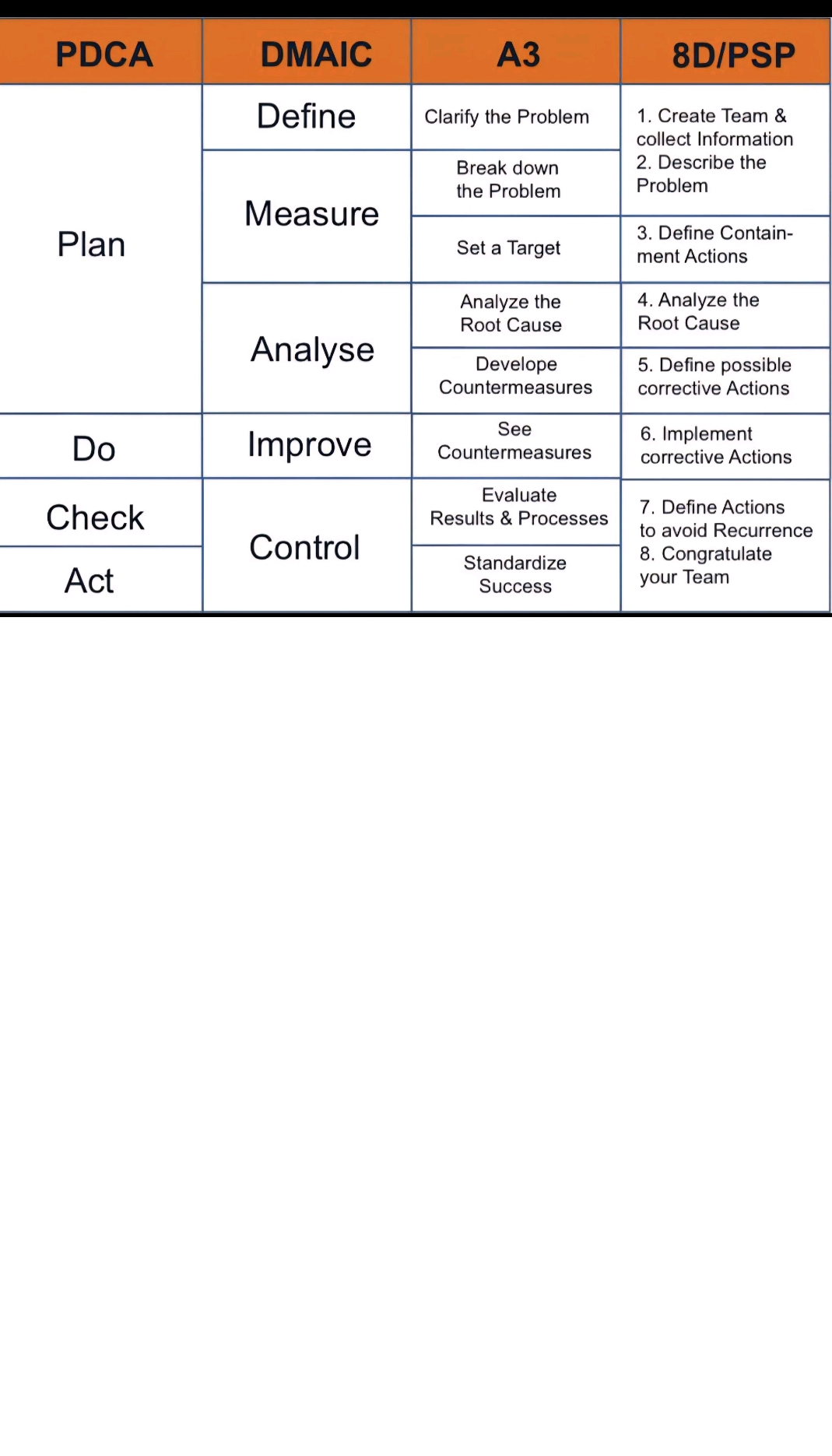

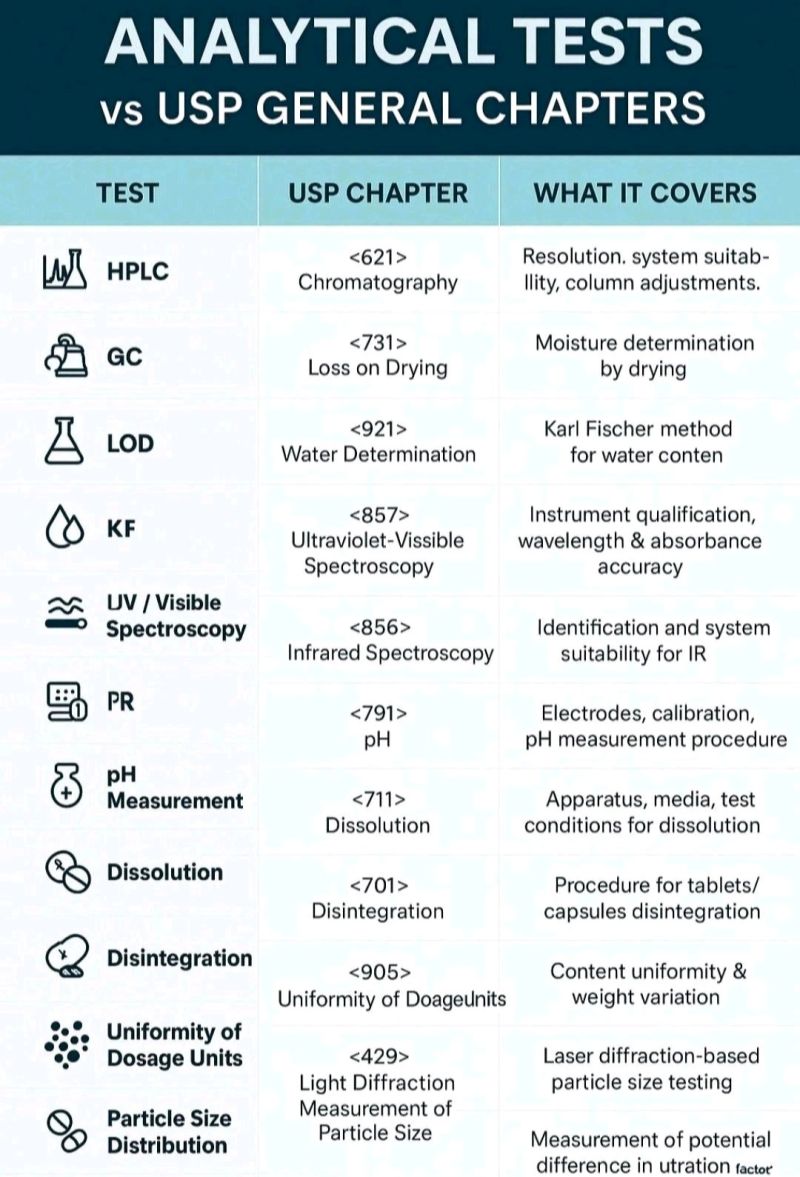
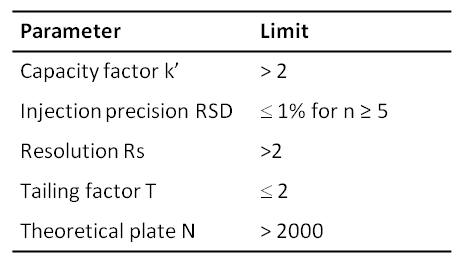
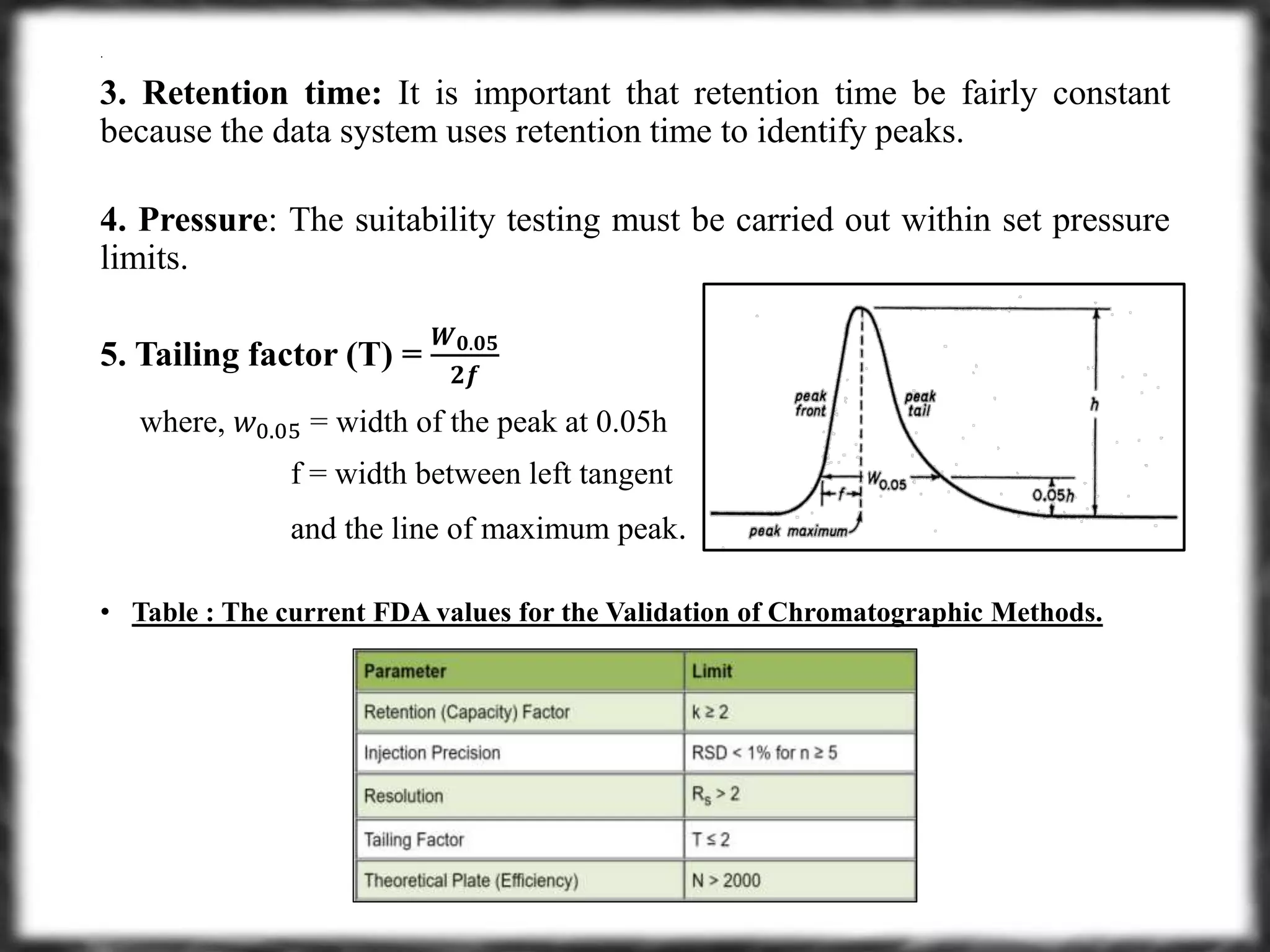
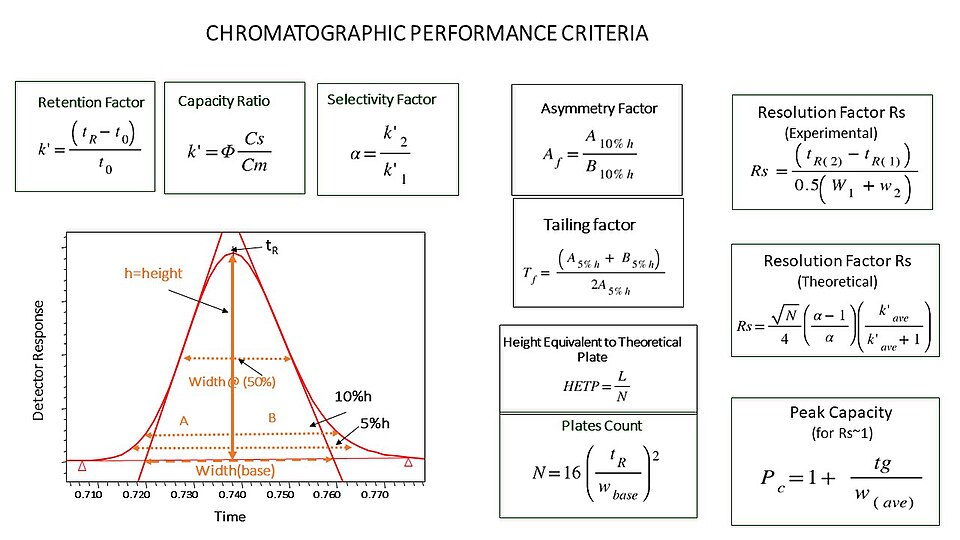
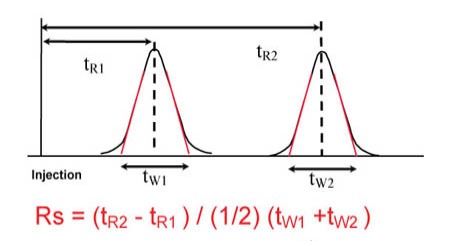
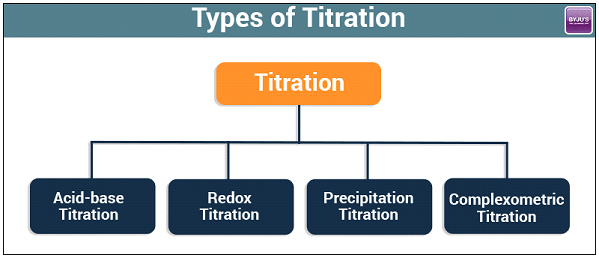
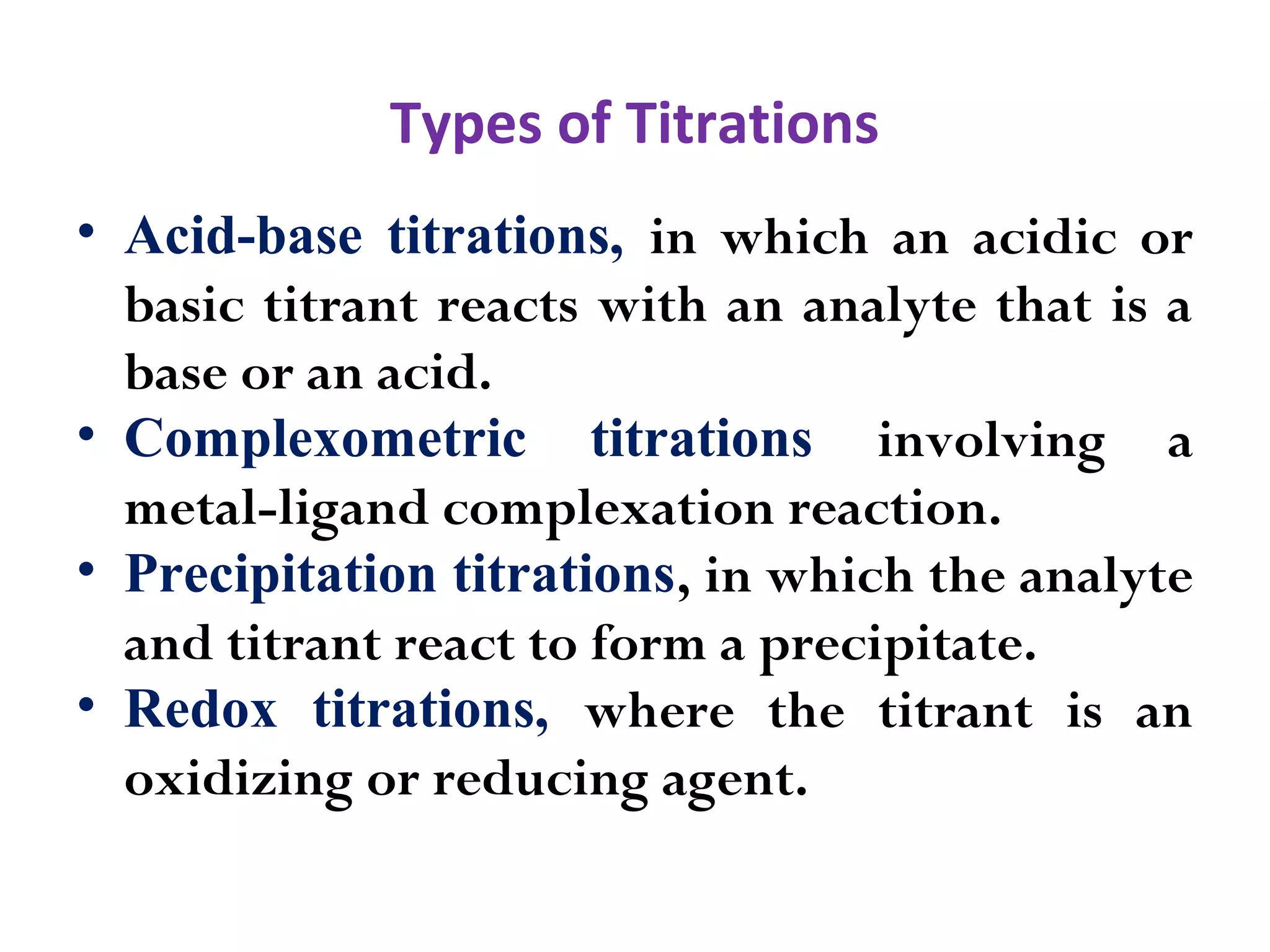
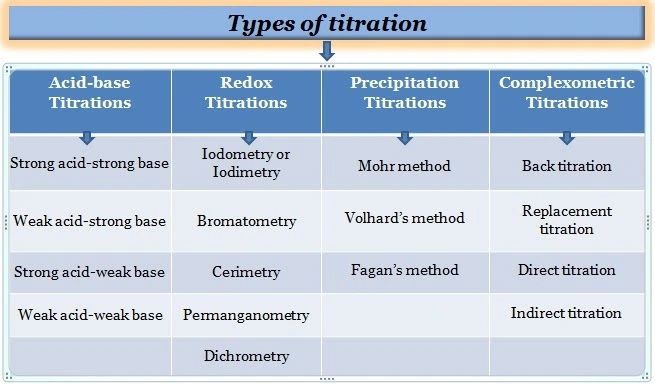
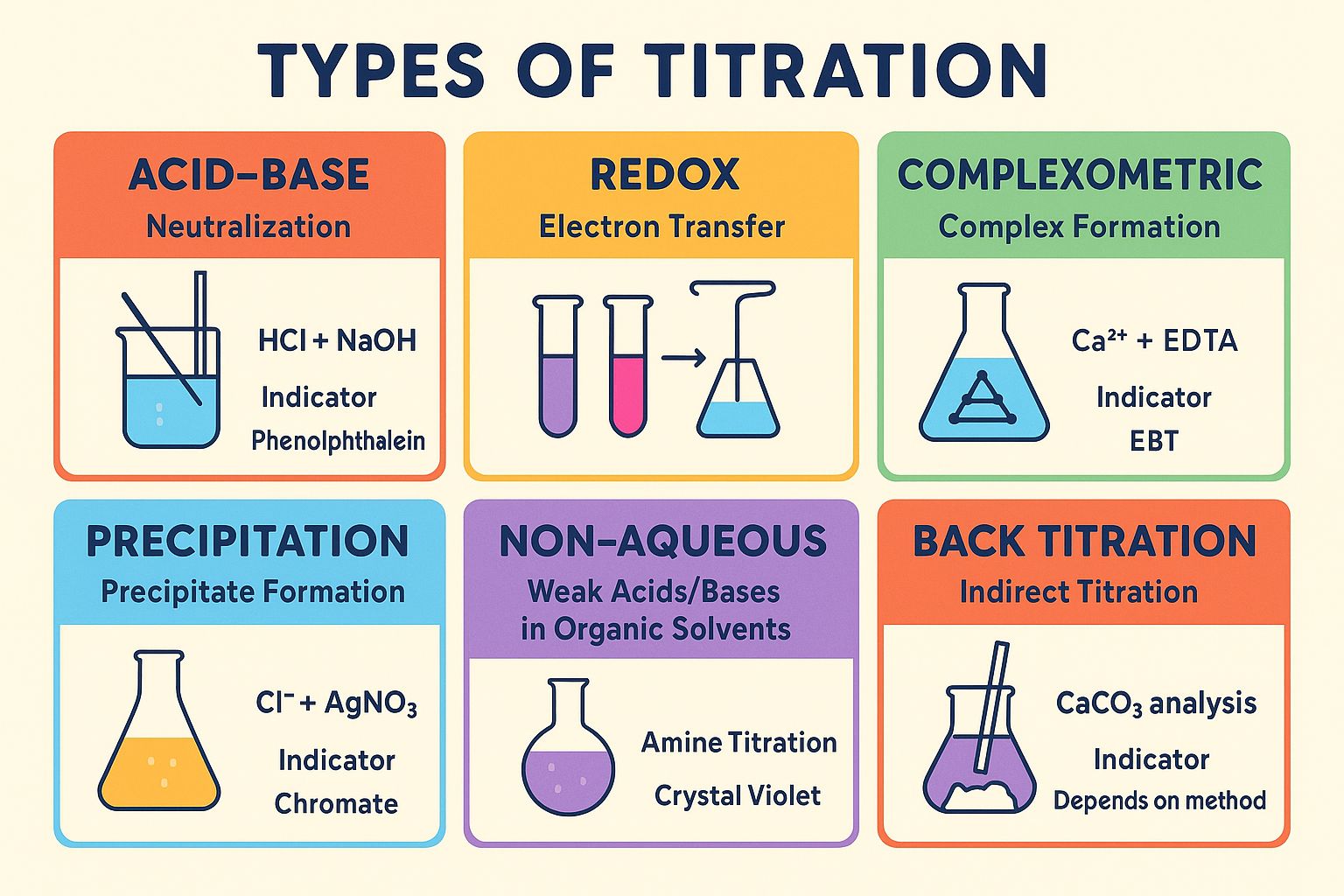
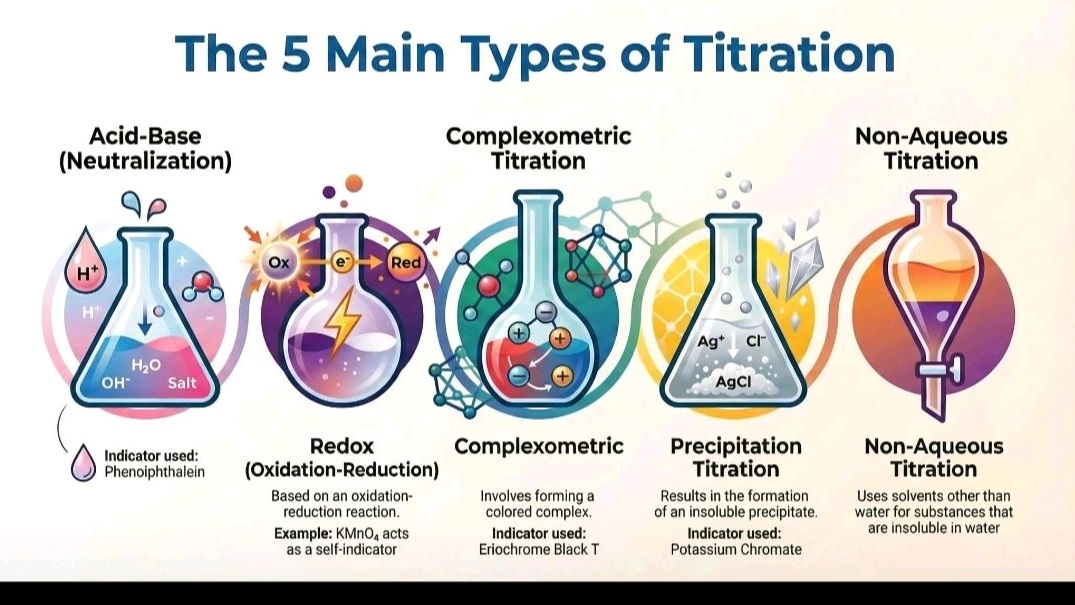
1.Acid-Base Titration
2. Redox Titrations
3. Precipitation Titration
4. Complexometric Titration