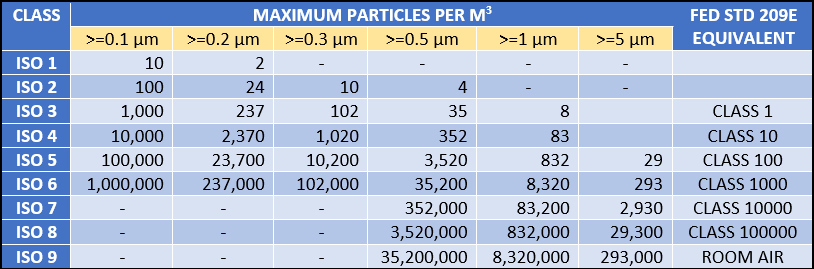
ISO Clean Air Standards-ISO 14644-1
ISO Clean Room Classification:-
Pharma Definition/Abbreviation, Pharma Beginners
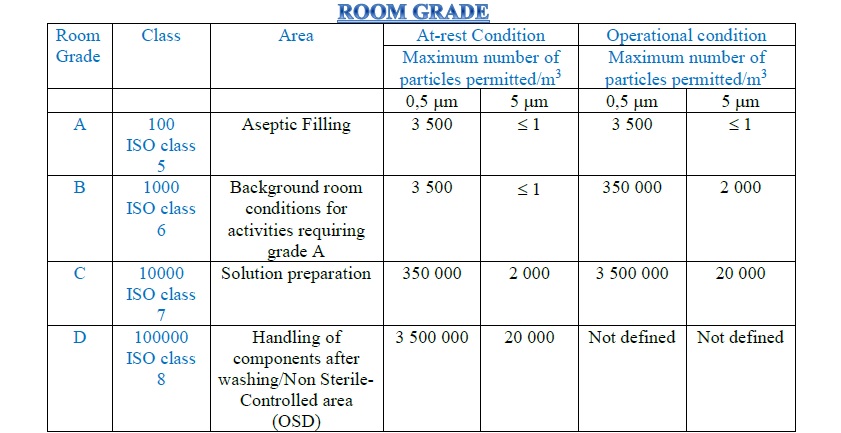
ISO Clean Room Classification:-


Reporting/Identification/Qualification of Impurities.
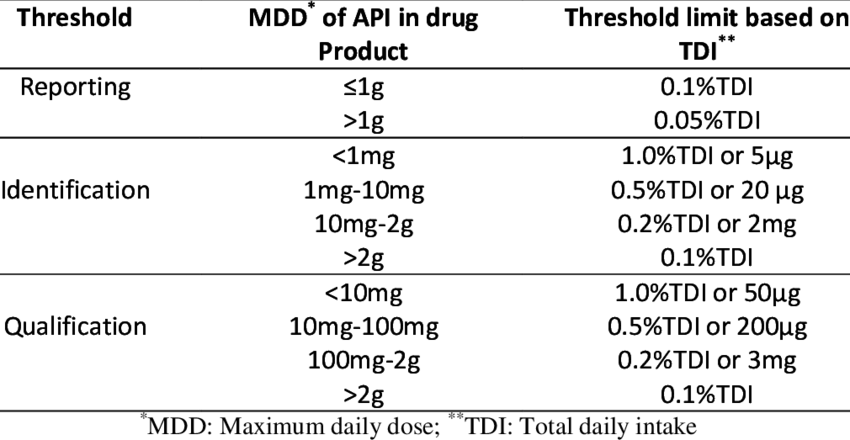
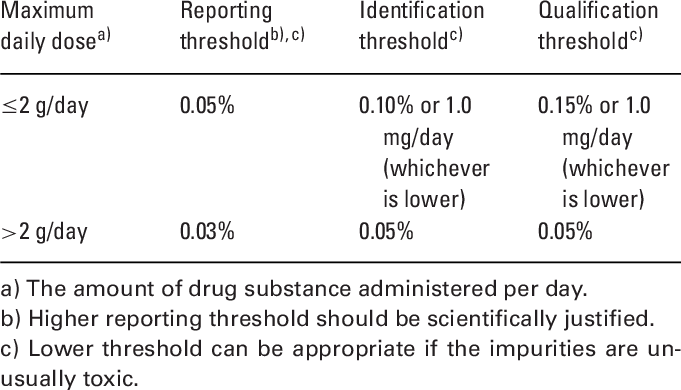
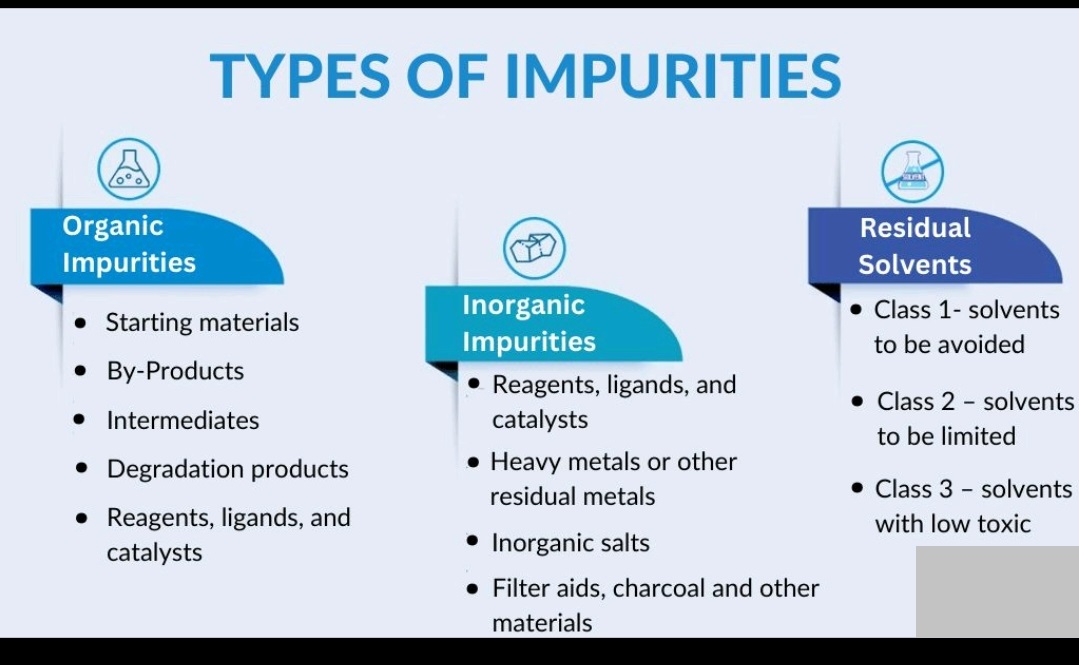
Types of Impurities- Organic Impurity (Degradation), In-organic impurity, Residual solvents (Class-I,II,III), elemental impurities, byproduct, heavy metals, reagents,ligands, salt, catalyst, toxic, process, leaching, Hydrolysis, Oxidation, reduction, stability, heat, light, contamination.

Elemental Impurities- Lead, Mercury,cadmium, Nickel,cobalt, arsenic. (USP-232,233)

Regulatory Definitions of Data Integrity
USFDA: “Data integrity refers to the completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded original, and accurate (ALCOA)”.
MHRA: “The extent to which all data are complete, consistent, and accurate throughout the data lifecycle.”
WHO: “Data integrity is the degree to which a collection of data is complete, consistent and accurate throughout the data lifecycle. The collected data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate”.
PICS: “Data Integrity is defined as the extent to which all data are complete, consistent, and accurate, throughout the data lifecycle”.
| Attributable | The Identity of the person completing a record (Who, When, Why). |
| Legible | The data is readable, Understandable, Traceable, Permanent allowing for a clear picture of the activities that occurred. |
| Contemporaneous | The data is recorded at the time it is generated or observed (No Back dating). |
| Original | Original Records must preserve data accuracy, completeness, content and meaning. Data as the file or format in which it was initially generated. |
| Accurate | The data record must be accurate whether paper or electronic, it must be exact, true and free from error (this might require a second verification if necessary). |
| Consistent | Consistent application of date and time stamps in the expected sequence. |
| Complete | All Information needs to be maintained. Batch pass-fail, Reanalyses carried out. (OOS, OOT). |
| Enduring | Medium used to record data should be permanent and not temporary memory RAM. |
| Available | Available/Accessible for review / audit for the life time of the record. |
| India | DCGI | Drug Controller General of India |
| India | CDSCO | Central Drugs Standard Control Organization |
| World | WHO | World Health Organization |
| USA | USFDA | U S Food and Drug Administration |
| UK | MHRA | Medicines and Healthcare products Regulatory Agency |
| EU | EMA | European Medicines Agency |
| EU | EDQM | European Directorate for Quality of Medicines |
| South Africa | MCC | The Medicines Control Council |
| Australia | TGA | Therapeutic Goods Administration |
| Japan | PMDA | Pharmaceutical Medical Devices Agency |
| Canada | TPD | Therapeutic Products Directorate |
| Singapore | HSA | Health Sciences Authority |
| Russia | Ministry of Health | |
| Brazil | ANVISA | Agência Nacional de Vigilância Sanitária |
| Switzerland | SWISSMEDIC | Swiss Agency for Therapeutic Products |
| Arab States | GCC | Gulf Cooperation Council |
Form 482: FDA may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a reported problem. The investigator will present credentials and “Notice of Inspection” upon arriving at your plant.
Form 483: An FDA form 483 is issued to firm management at the conclusion of an inspection when an investigator has observed any conditions that in their judgement may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related acts.
CDSCO: The Central Drugs Standard Control Organisation is India’s national regulatory body for pharmaceuticals and medical devices. The Drug Controller General of India (DCGI) regulates pharmaceutical and medical devices and is positioning within the Ministry of Health and Family Welfare.
EMA: The European Medicines Agency is an agency of the European Union in charge of the evaluation and supervision of medicinal products.
PIC/S: Pharmaceutical Inspection Convention/ Pharmaceutical Inspection Co-operation Scheme
The main aim of PIC/S is to improve the Co-operation in the field of GMP between regulatory authorities and pharmaceutical industry.
PIC was founded in 1970 by European Free Trade Association (EFTA), Because of incompatibility between convention and European law, it was not possible for new countries to be admitted as members of PIC. As a consequence, the Pharmaceutical Inspection Co- operation Scheme was formed on 2 November 1995.
ICH: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is unique in bringing together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceuticals and develop ICH guidelines.
ICH Products: Quality, Safety, Efficacy, Multidisciplinary
WHO: The World Health Organization is a specialized agency of the United Nations responsible for international public health. The WHO Constitution, which establishes the agency’s governing structure and principles, states its main objective as “the attainment by all peoples of the highest possible level of health”
Regulatory Affairs: Regulatory Affairs in a Pharmaceutical industry, is a profession which acts as the interface between the pharmaceutical industry and Drug Regulatory authorities across the world. It is mainly involved in the registration of the drug products in respective countries prior to their marketing.
Validation: Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and Quality Attributes. (Validation is Process Oriented)
Verification: Verification is the act or process of establishing the truth or reality of something.
Qualification: Is an act or process to assure something complies with some condition, standard or specific requirements.