Topic:– Method Transfer
Definition:
The formal transfer of a test methodology is a required GMP process that qualifies a receiving laboratory to utilize a method that originated in a transferring laboratory.
This process is intended to ensure and document that the method performs as intended within the receiving laboratories environment.
Guidelines – 1
–USP <1224>TRANSFER OF ANALYTICAL PROCEDURES
–PDA TR57
–PDA TR65
–WHO guideline, No.961 Annex 7
–FDA Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics
The transfer of analytical procedures (TAP), also referred to as method transfer, is the documented process that qualifies a laboratory (the receiving unit) to use an analytical test procedure in another laboratory (the transferring unit), thus ensuring that the receiving unit has the procedural knowledge and ability to perform the transferred analytical procedure as intended.
In case of method transfer between two laboratories, different approaches may be taken to achieve the successful transfer of the procedure.
The approach is method transfer from the originating laboratory to the receiving laboratory.
The originating laboratory is defined as the laboratory that has developed and validated analytical method.
The receiving laboratory is defined as laboratory to which the analytical method to be transferred and that will participate in the method transfer studies.
In the method transfer, it is recommended that protocol be initiated with details of the experiments to be performed and acceptance criteria (in term of the difference between the means of the two laboratories) for passing the method transfer.
-
Sending Unit :- (SU)
Laboratory which the method originates from Development lab, routine QC lab, Originating lab, sending lab, transferring lab.
-
Receiving Unit:- (RU)
Laboratory which is being qualified on the validated method CRO, CMO, commercial unit, Receiving lab, transfer site.
Site Responsibilities :- |
|||
Sending Unit (SU) |
Receiving Unit (RU) |
||
Readiness assessment |
Review transfer package |
||
Compile data/history |
Evaluate internal gaps |
||
Organize training |
Define training requirements |
||
Prepare transfer package |
Provide resources |
||
Draft protocol/set acceptance criteria |
Perform transfer and evaluate siteresults |
||
Provide samples |
Provide data to SU |
||
Provide troubleshooting support |
Approve transfer report |
||
Compare RU data against SU data |
|||
Approve transfer report |
|||
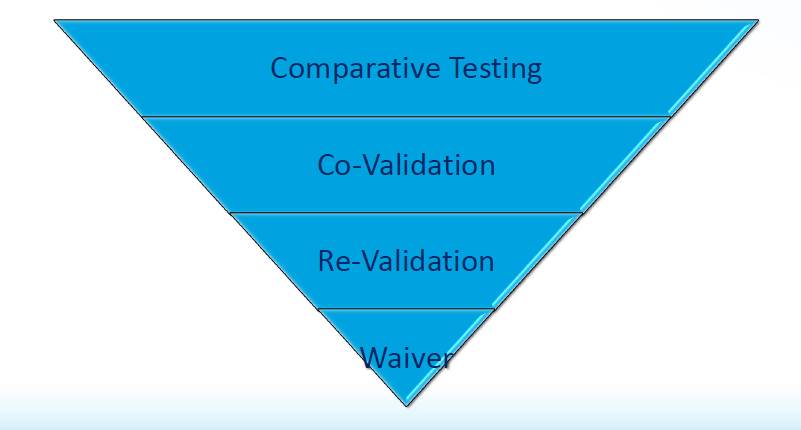
Types of method transfer:-
Method transfer is defined as the process that qualifies a laboratory to use an analytical test procedure. The most common variations of method transfer are comparative testing, co-validation between two laboratories or sites, complete or partial method validation or revalidation, and the omission of formal transfer, sometimes termed the transfer waiver.
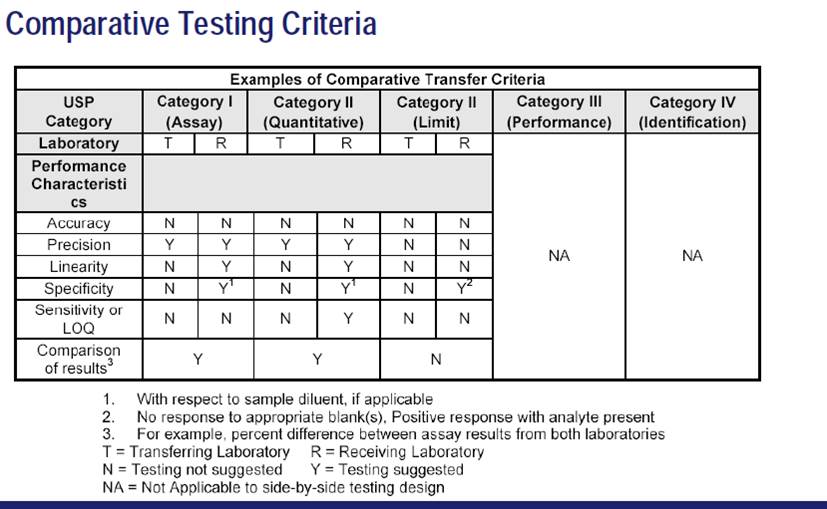
Comparative Testing:-
Comparative testing is the most common form of method transfer in the pharmaceutical industry.
It involves two or more laboratories or sites executing a preapproved protocol that details the criteria by which the receiving laboratory is deemed to be qualified to use the method(s) being transferred. The resulting data are compared with a set of predetermined acceptance criteria. Comparative testing also is used in other scenarios during development and Post approval, including other manufacturing sites and contract research organizations.
Co-validation:-
Between two laboratories, An alternative to comparative testing is to involve the receiving laboratory in the validation of the method to be transferred. By definition, a laboratory or site that performs validation experiments is qualified to run that method routinely. To perform such a transfer, one must identify which validation parameters are to be generated or challenged by the sending and receiving laboratories. A reasonable approach is to involve the receiving laboratory in the inter laboratory qualification, there by generating a matrix of data that summarizes the suitability of the testing site, analyst, date of analysis, and instrumentation for exercising the analytical test procedures. By fully describing the experimental design for the validation exercise and including the resulting data in the method validation report, it is possible to have this document stand as proof of transfer of the analytical test procedure.
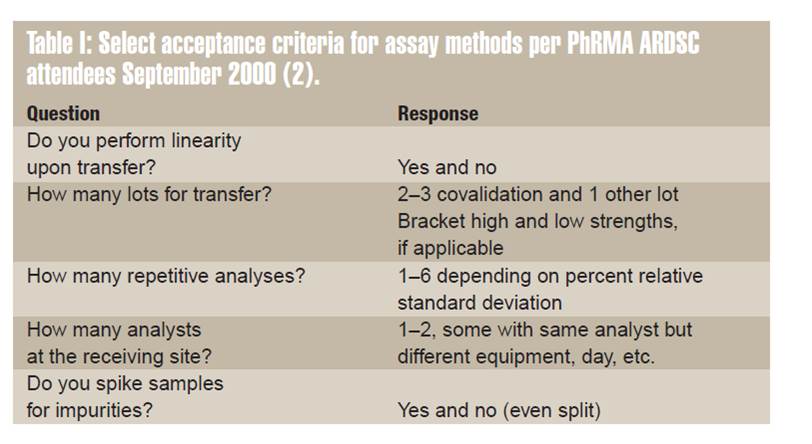
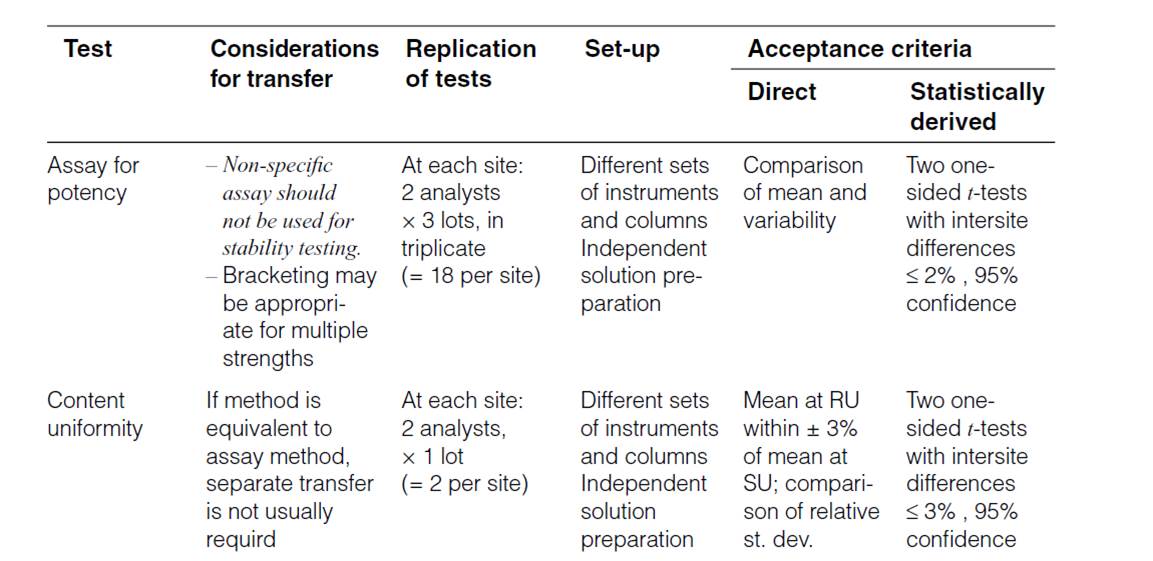
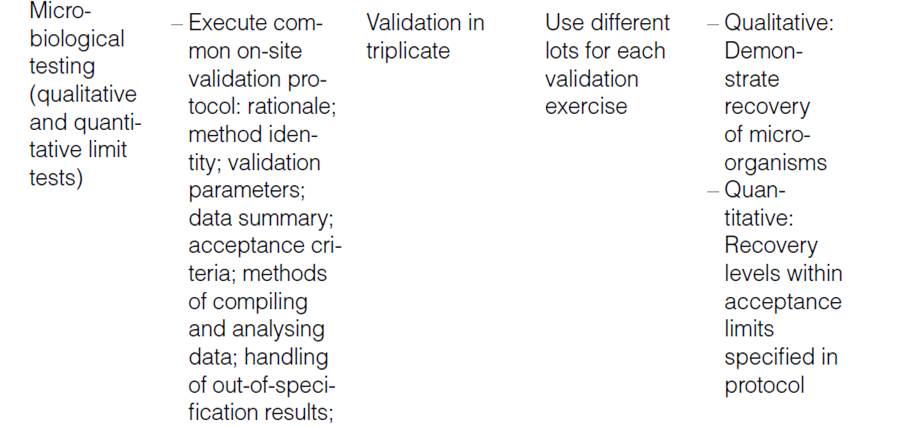
Method validation and/or Revalidation:-
Another option for accomplishing method transfer is for the receiving laboratory to repeat some or all of the validation experiments. After validation is completed, the second laboratory is deemed qualified to perform the method. The choice of validation parameter(s) depends highly on the type of method being transferred. For example, content uniformity assays to determine consistency of product potency depend heavily on the precision of the method.
Method validation and/or revalidation:-
Another option for accomplishing method transfer is for the receiving laboratory to repeat some or all of the validation experiments. After validation is completed, the second laboratory is deemed qualified to perform the method. The choice of validation parameter(s) depends highly on the type of method being transferred. For example, content uniformity/assays to determine consistency of product potency depend heavily on the precision of the method.
Transfer waiver:-
Certain situations might warrant omitting conventional transfer qualification experiments altogether. When a transfer waiver is applied, the receiving laboratory can proceed to use the analytical test procedures under discussion without generating inter laboratory comparative testing data.
To proceed without generating such data, one must document the reasons why this action was taken. Examples of these reasons were discussed by PhRMA workshop attendees; they include the following:
-
The receiving laboratory already is testing the product and is thoroughly familiar with the procedure(s).
-
The new dosage form possesses either a comparable composition or concentration of active pharmaceutical ingredient relative to the existing product.
-
The analytical method(s) are the same or very similar to the methods that are already in use.
-
The method validation package encompasses the new methods.
-
Personnel who developed the methods move from one site or laboratory to another as the project
-
The new methods involve changes that do not substantially alter the ability to use the method (e.g., changes in sample preparation procedures or changes in calculation formulas).



Disclaimer:-This presentation is solely prepared for training, sharing knowledge and information purpose only collected from various guidelines and literature. The information presented here is only for reference purpose.
