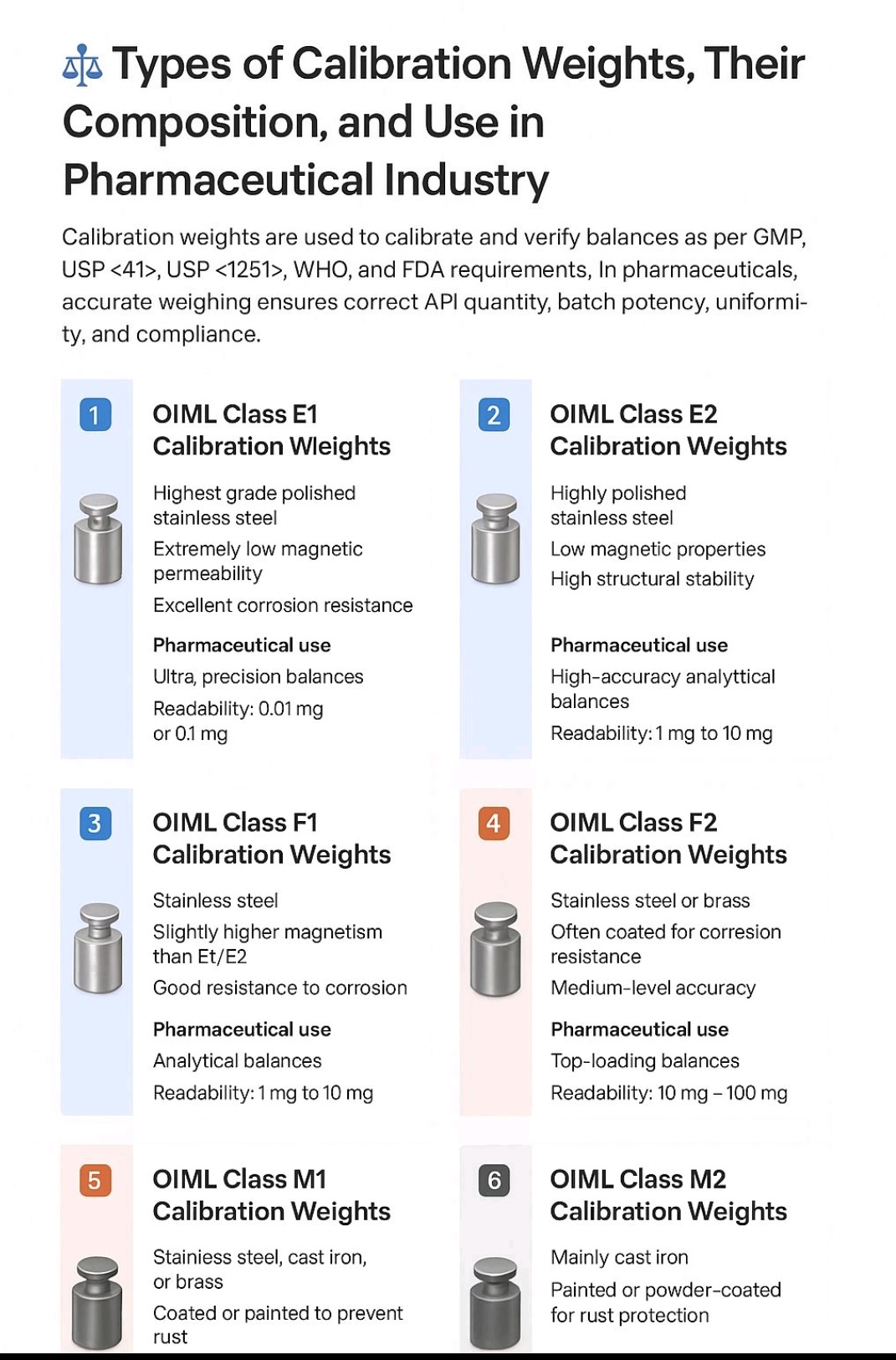
Types of Calibration Weights
Pharma Definition/Abbreviation, Pharma Beginners

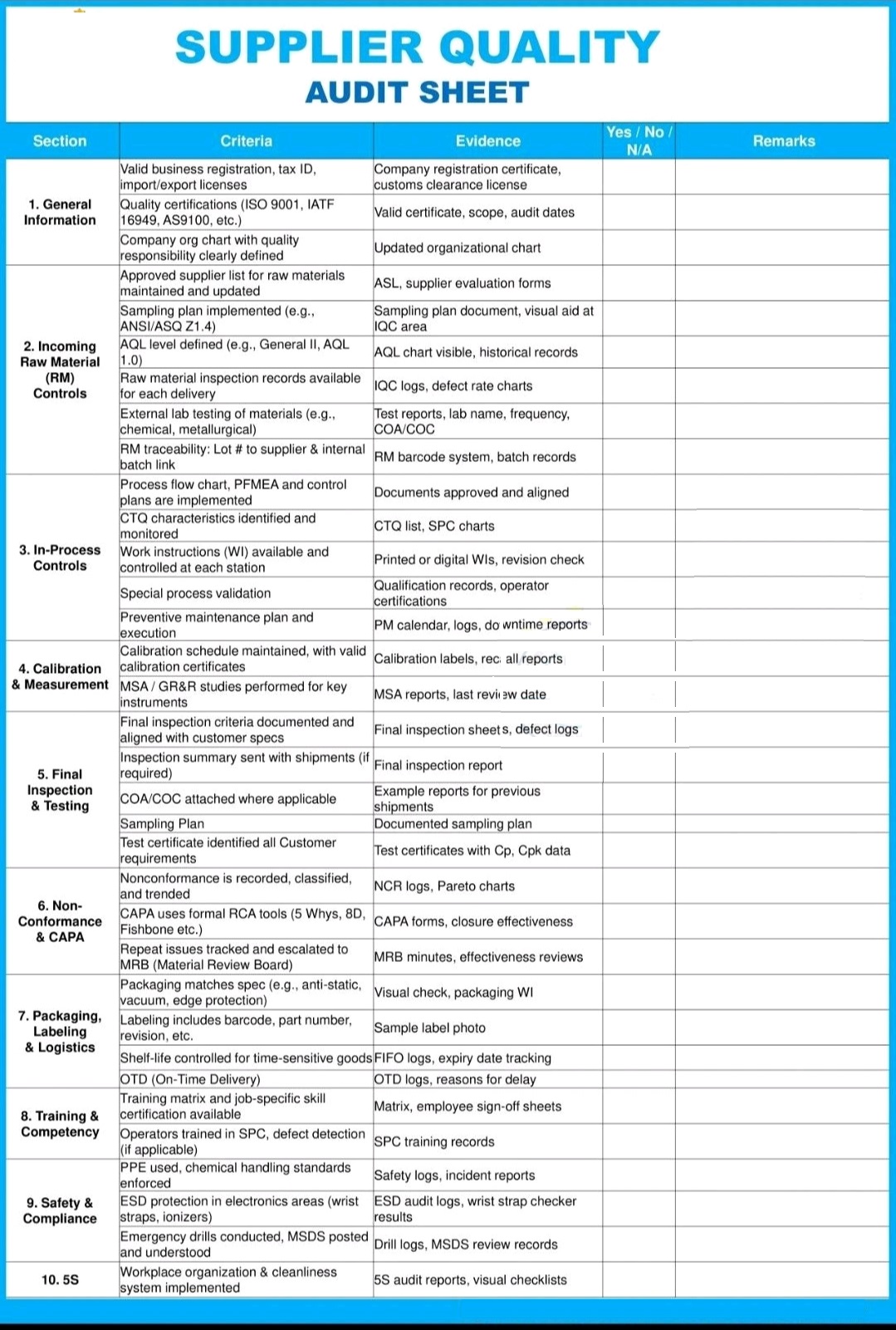
The vendor selection involves a rigorous assessment of a vendor’s capabilities, manufacturing processes, and quality systems, and it includes ongoing monitoring and re-evaluation to minimize risks like product recalls, adverse events, or patient harm due to poor-quality materials.
Vendor qualification in pharma is a systematic process of evaluating and approving suppliers to ensure they meet stringent quality, safety, and regulatory standards for materials and services.



Nitrogen purging is an industrial process where undesirable gases, moisture, or other contaminants are removed from a system by displacing them with inert nitrogen gas.

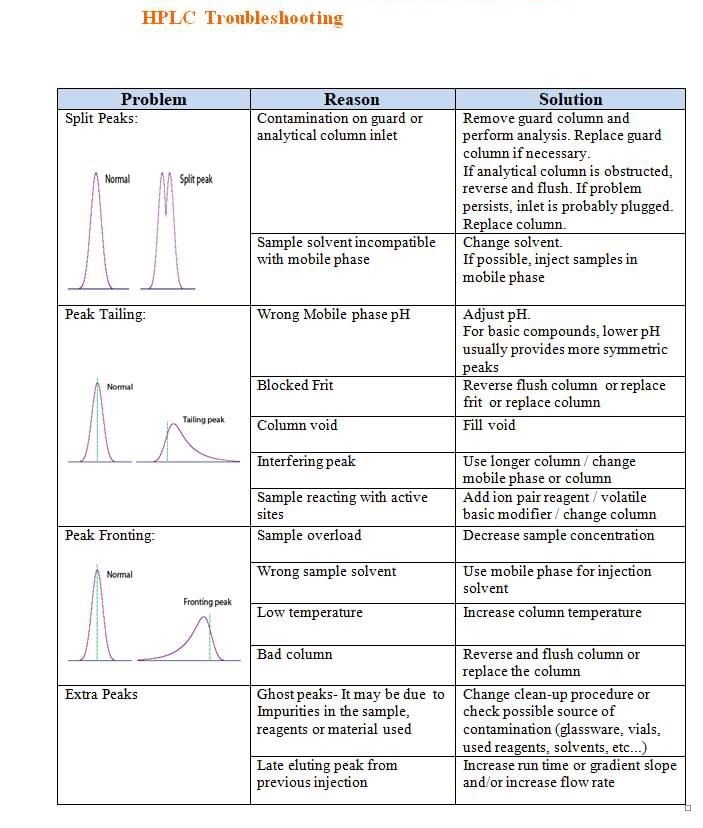
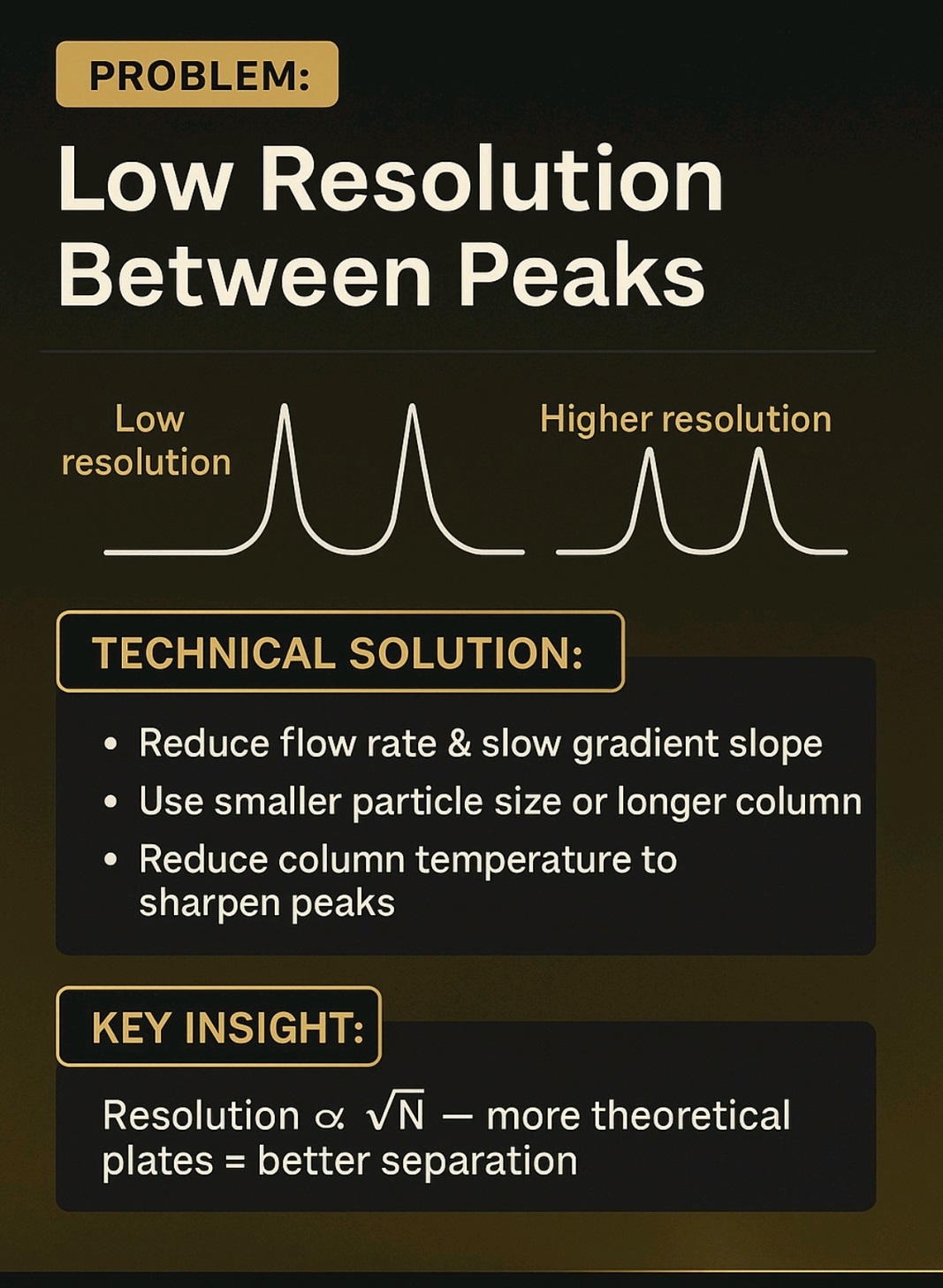
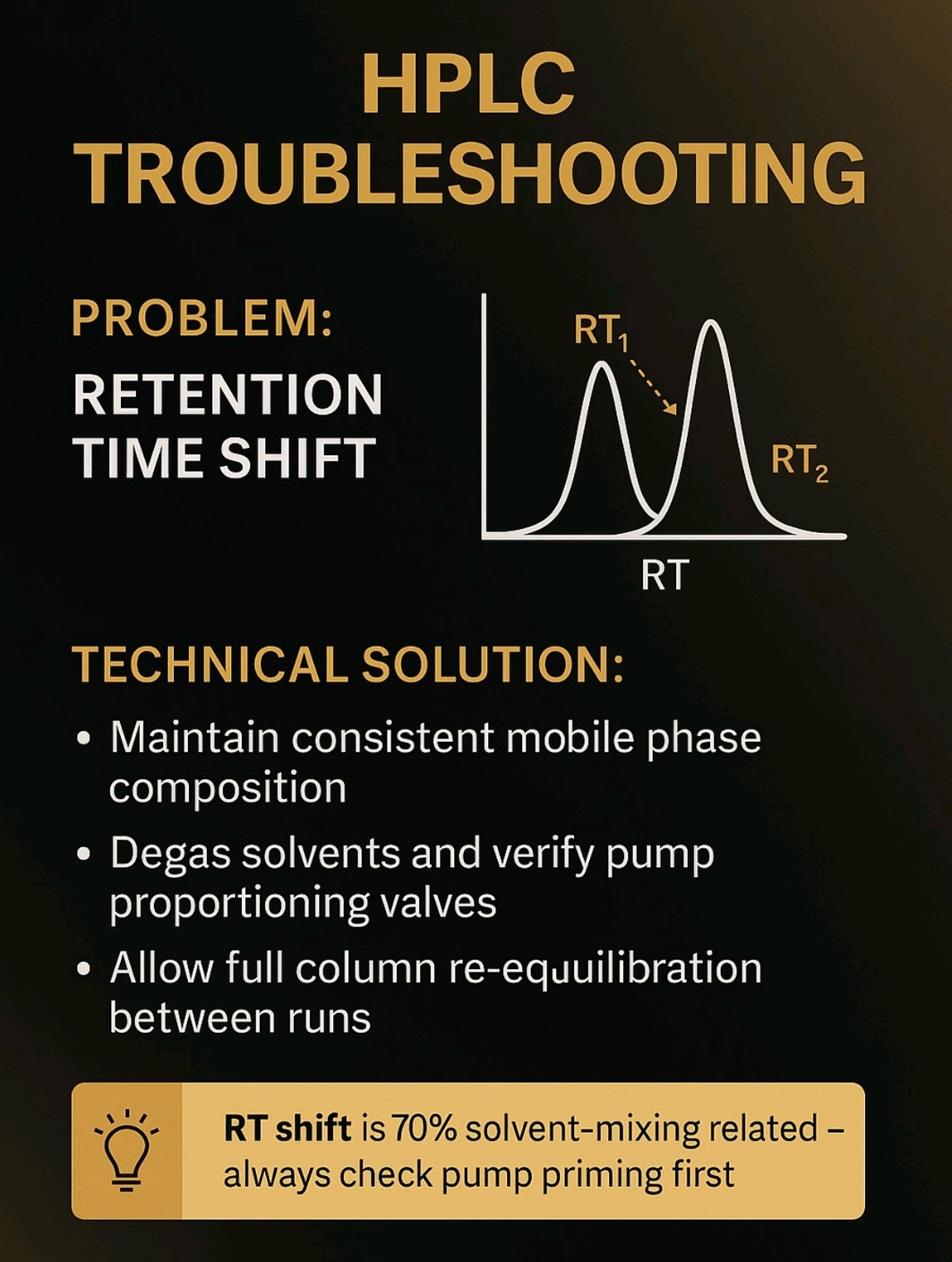
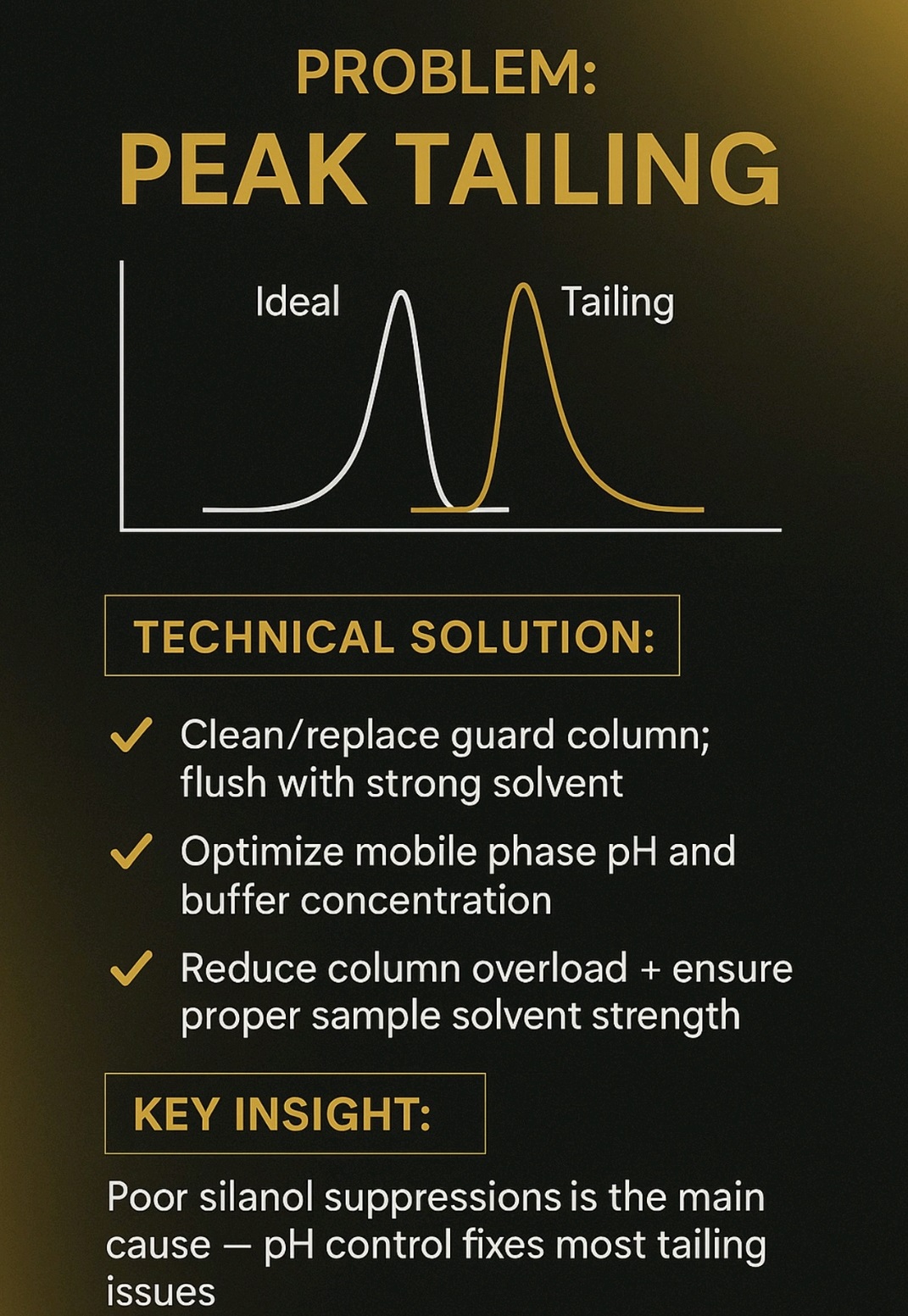
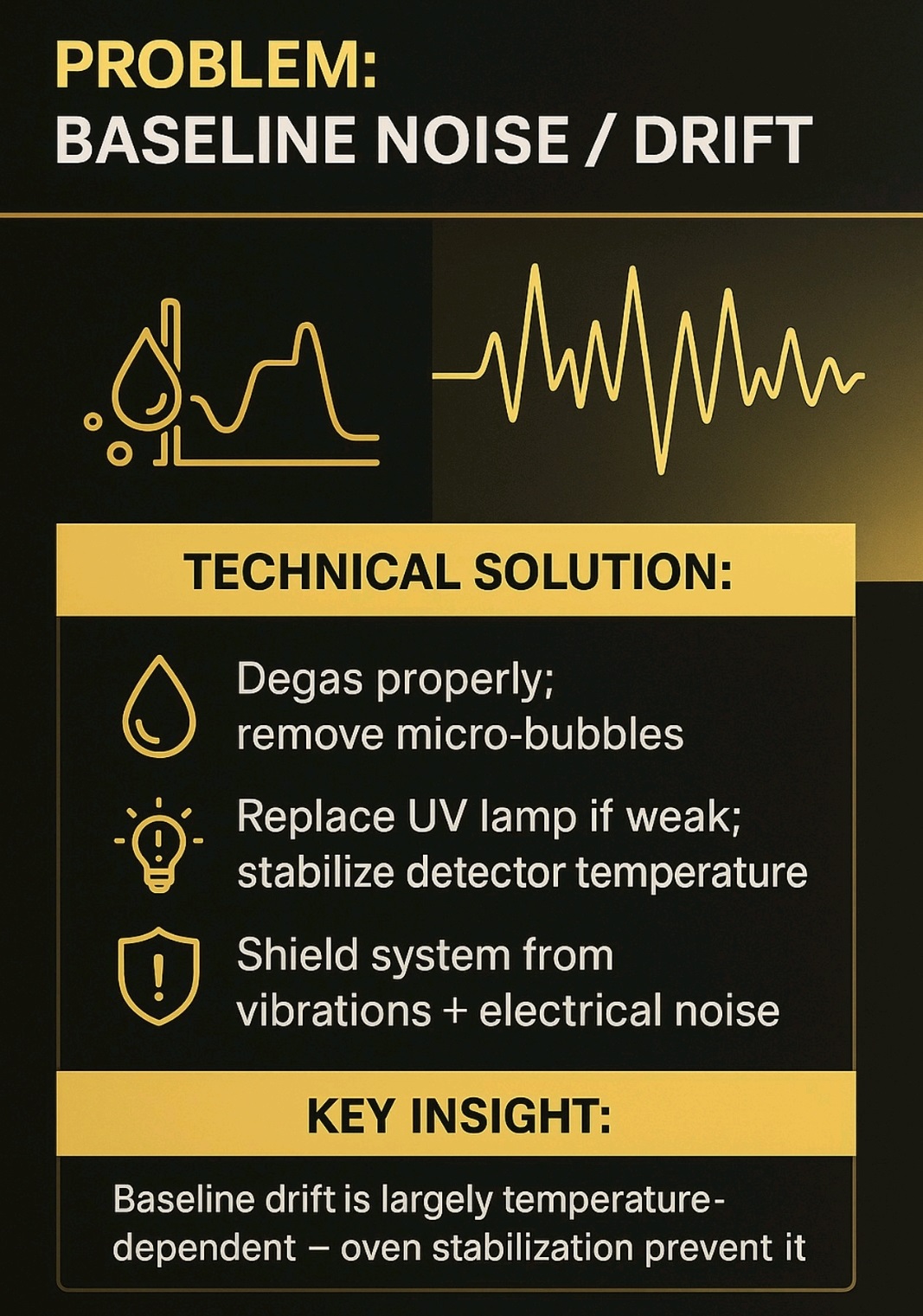
High Back pressure, Noisy baseline, carry over, Noise, Retention time (RT) shift, fronting, Tailing, Ghost Peak, low Resolution, Baseline Drift, Air bubble, Degassing, Buffer, pH, HPLC Column, Theoretical Plates, Frits, Mobile Phase, solvents, leakage, Injector, Lamp energy, calibration, Purge, flush, washing, filter,pump, blockage, C18,C8, storage, Flow, Normal phase, reverse phase, Needle, injector loop, composition, Gradient, Guard column, standard, sample, overload, concentration, system suitability, Methanol, Acetonitrile.



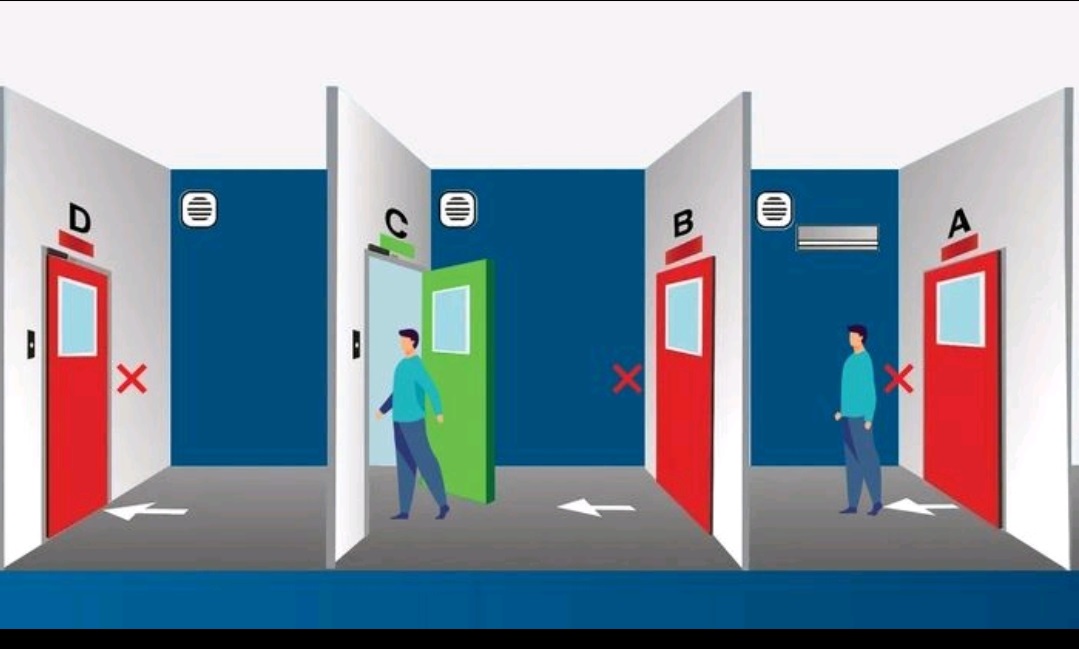
Door Interlock
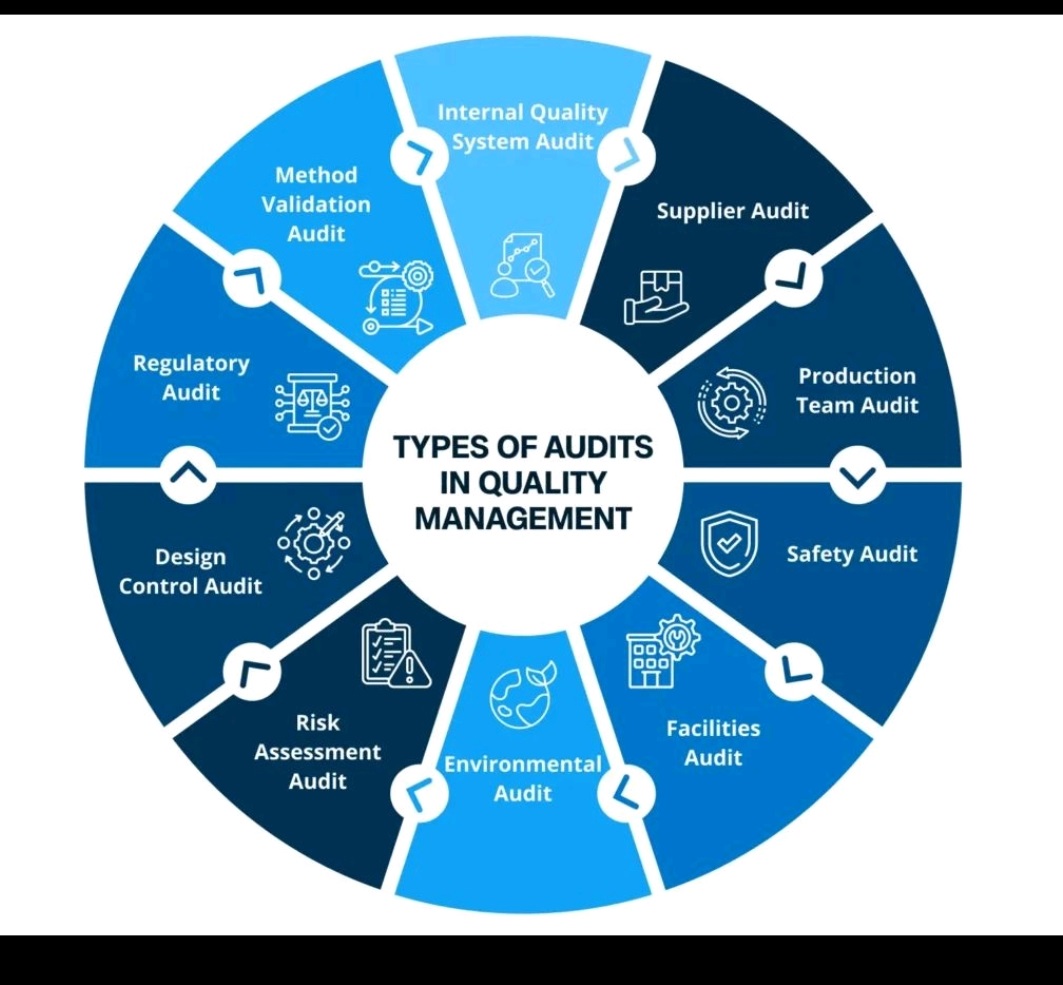
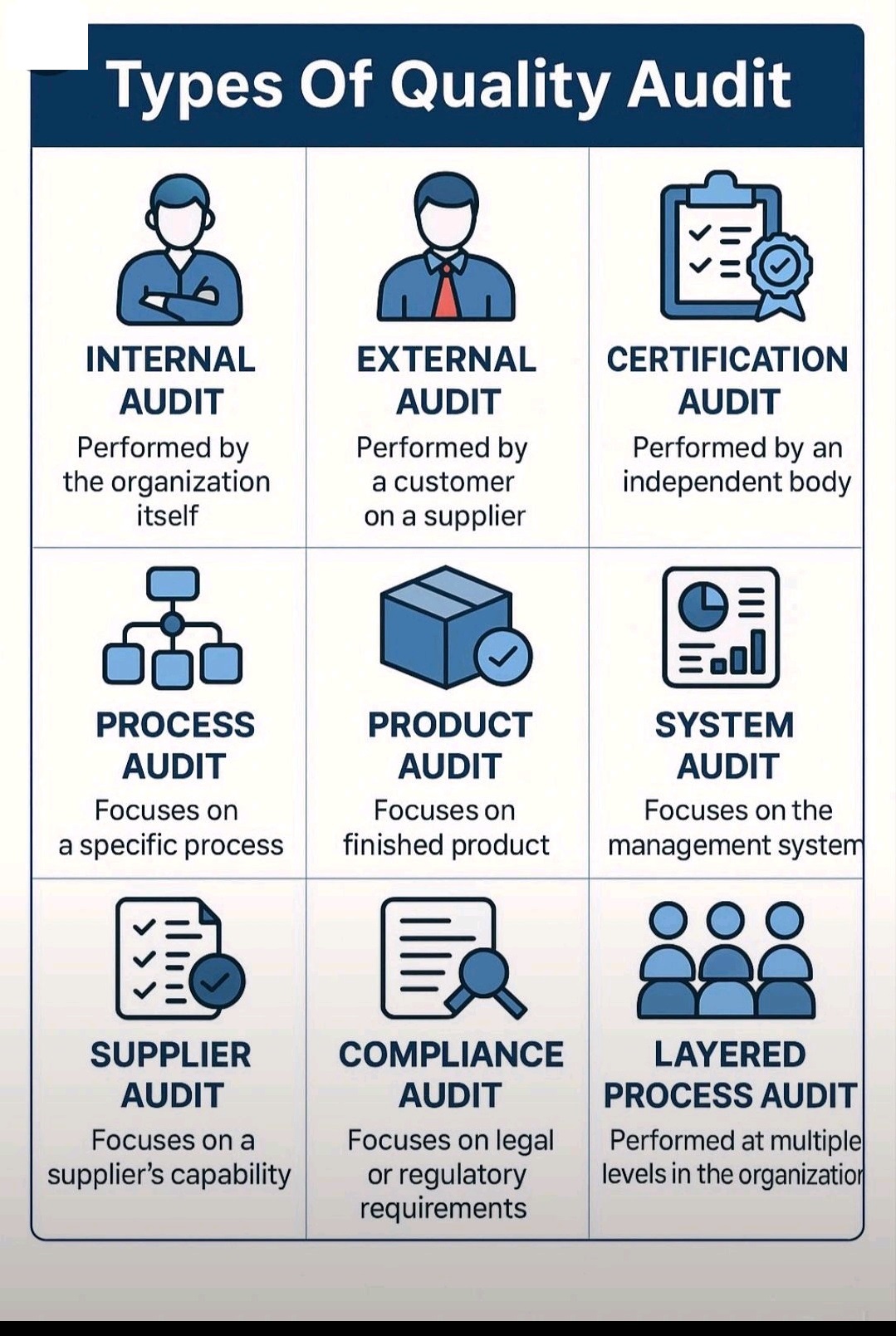
Verification Vs Calibration Vs Qualification Vs Validation

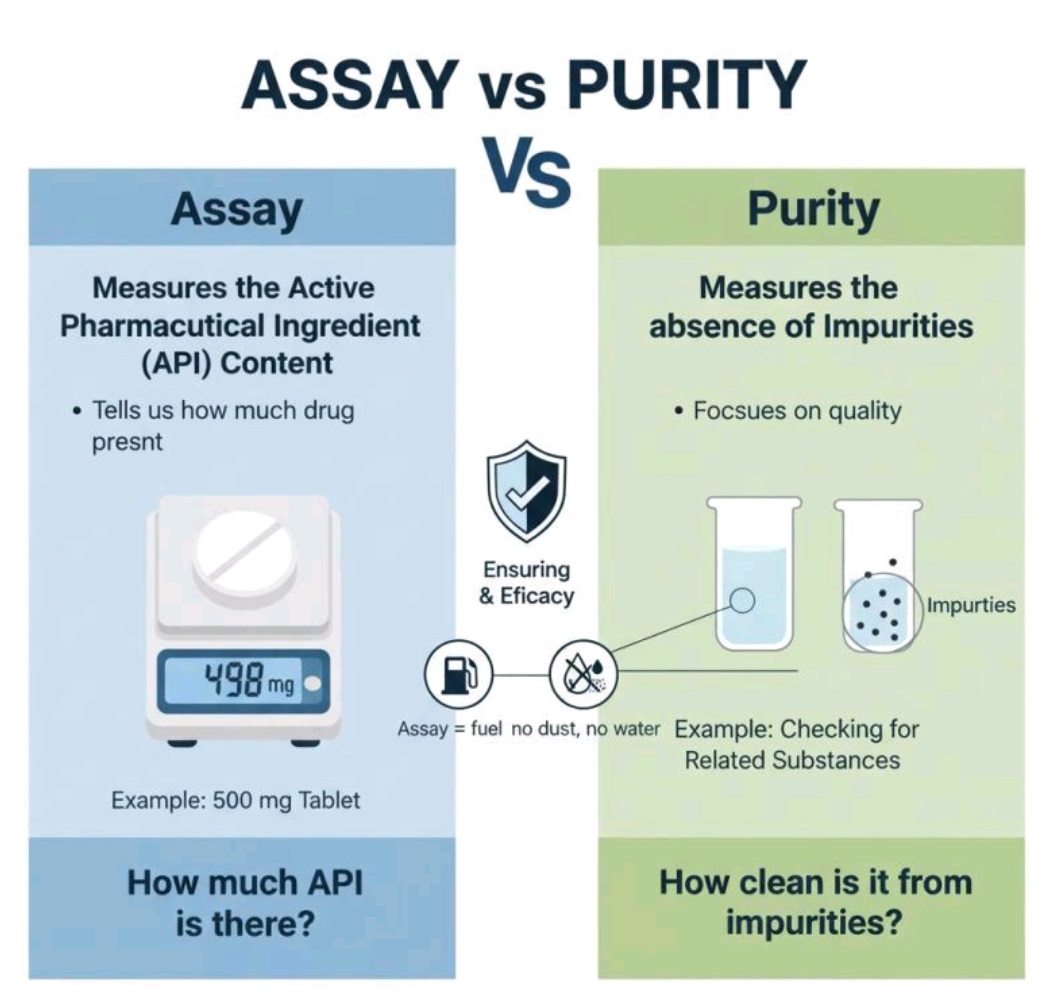
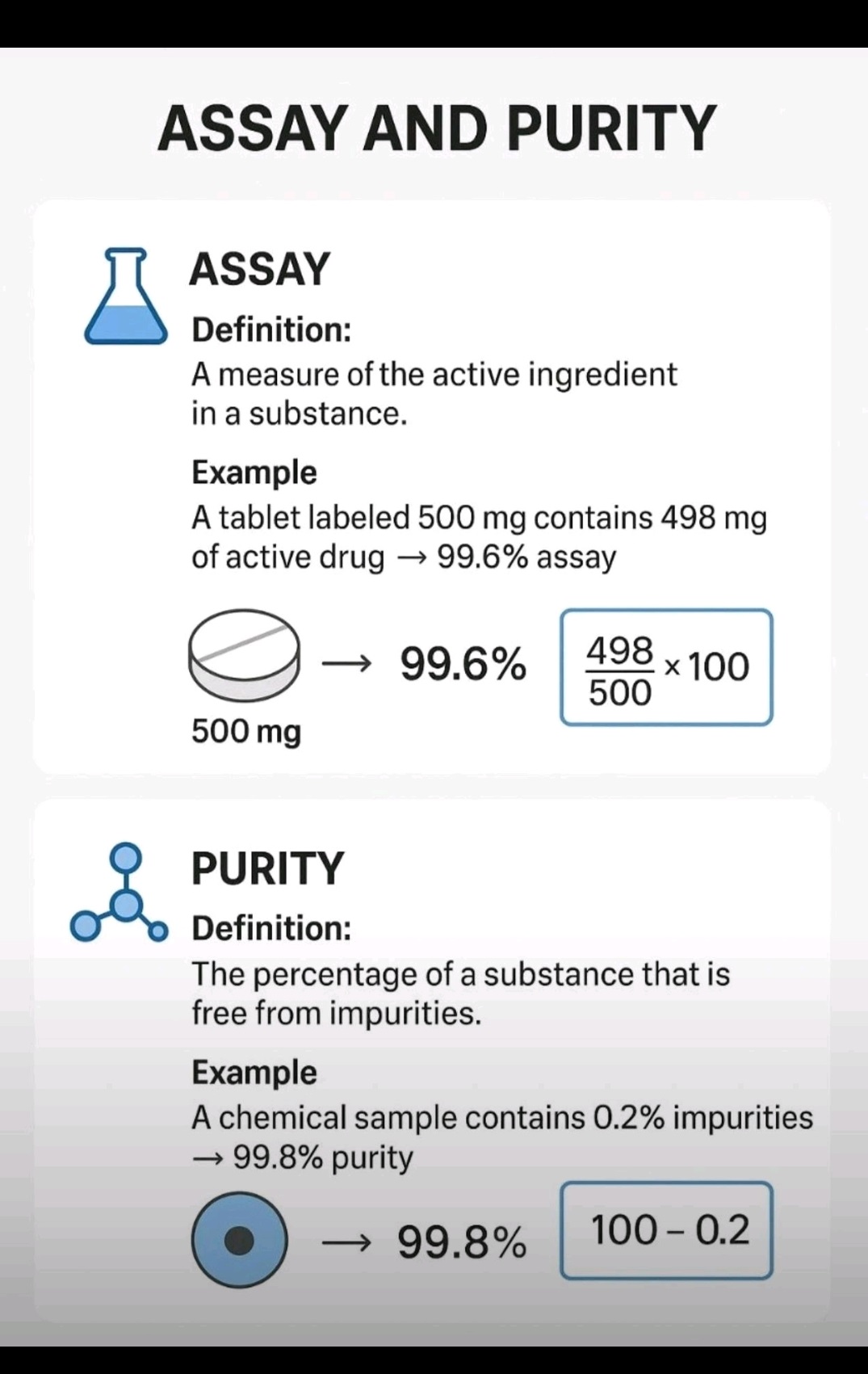
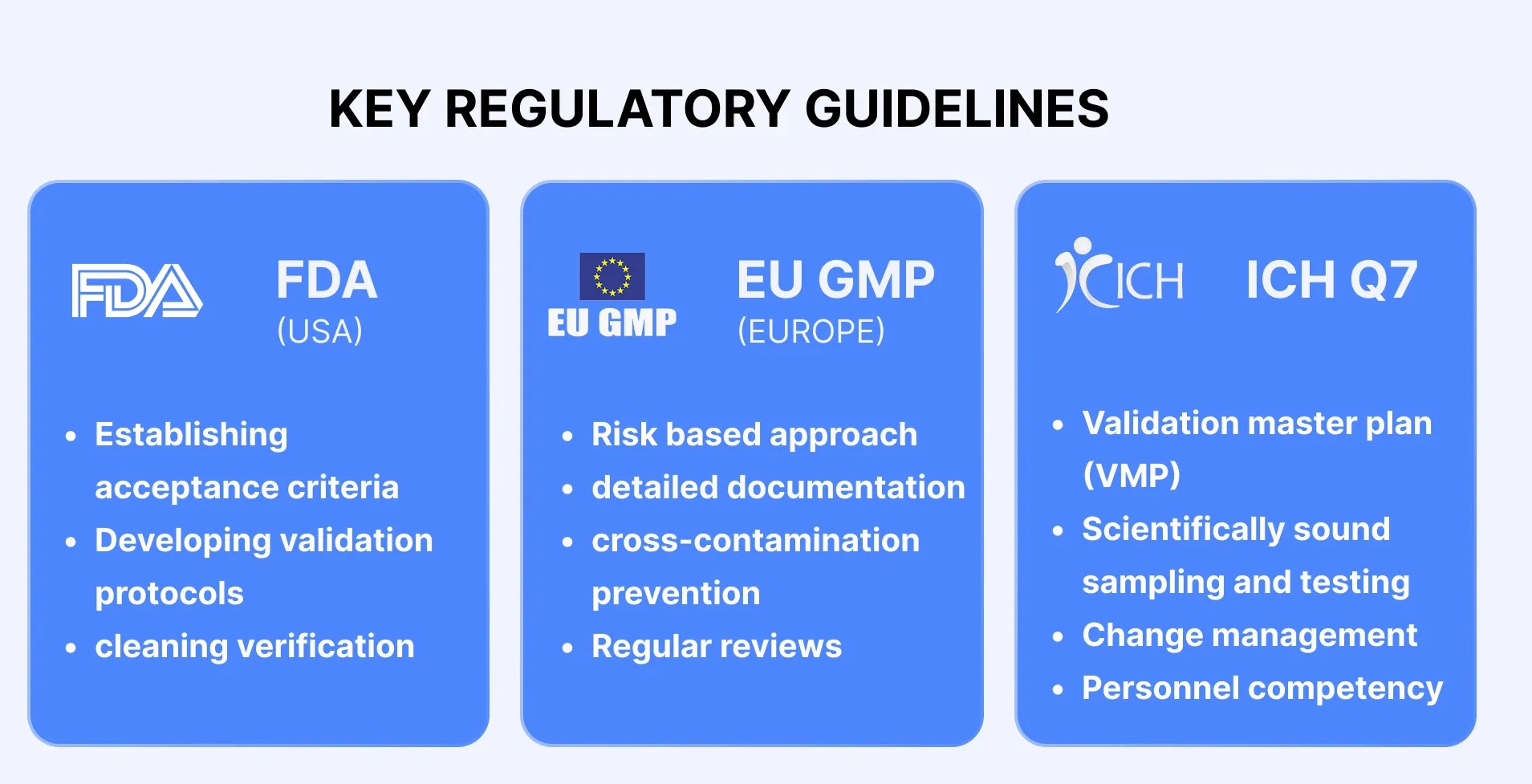
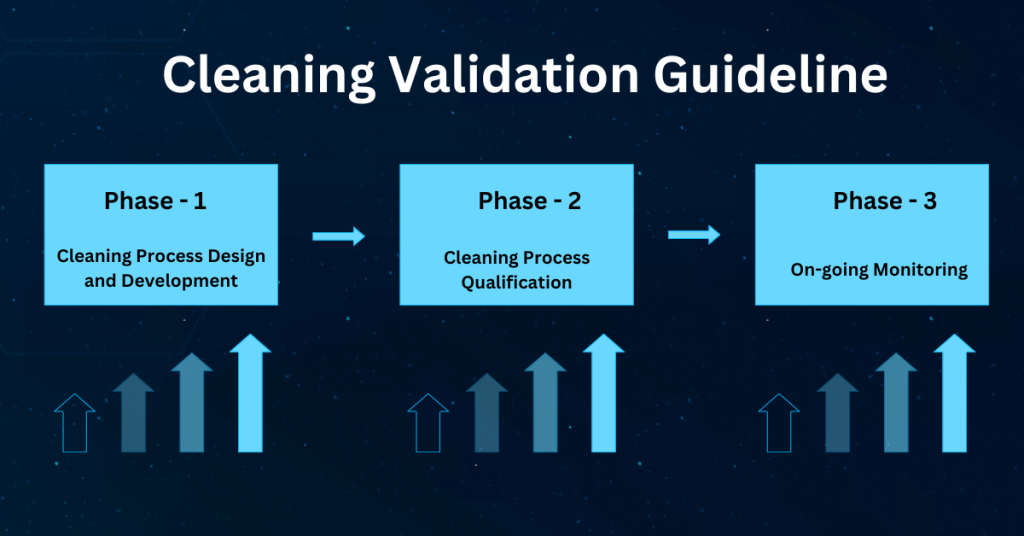
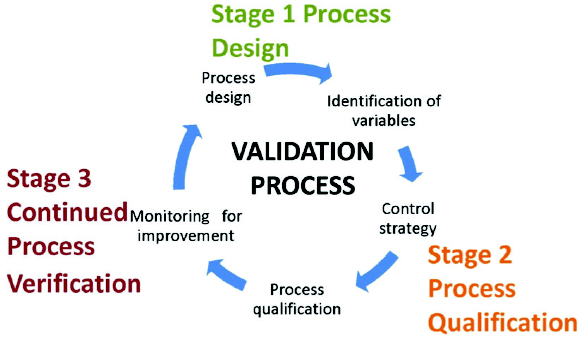
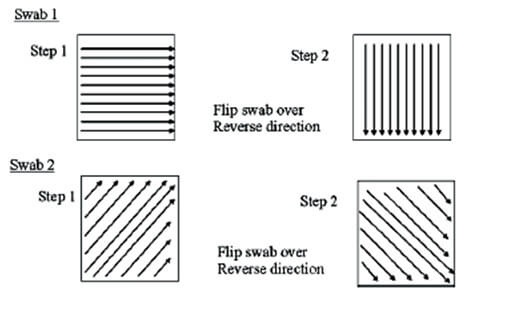
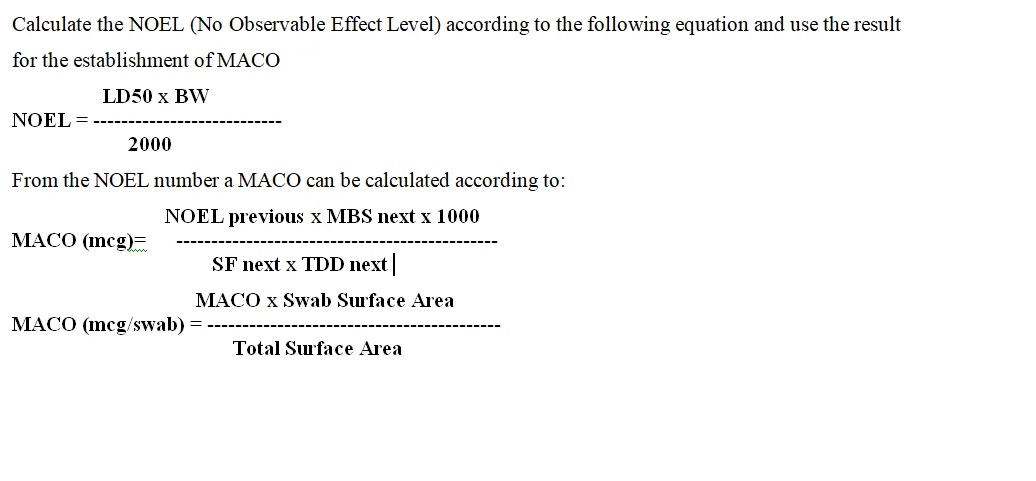
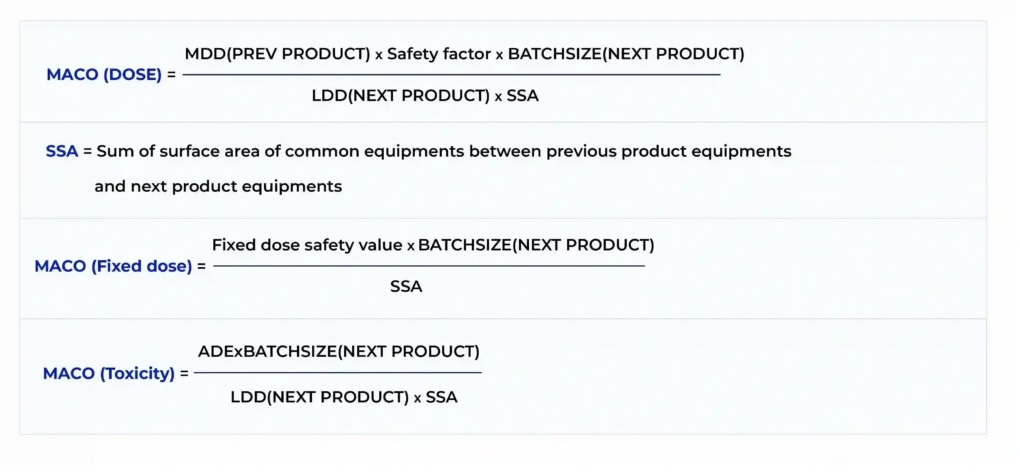
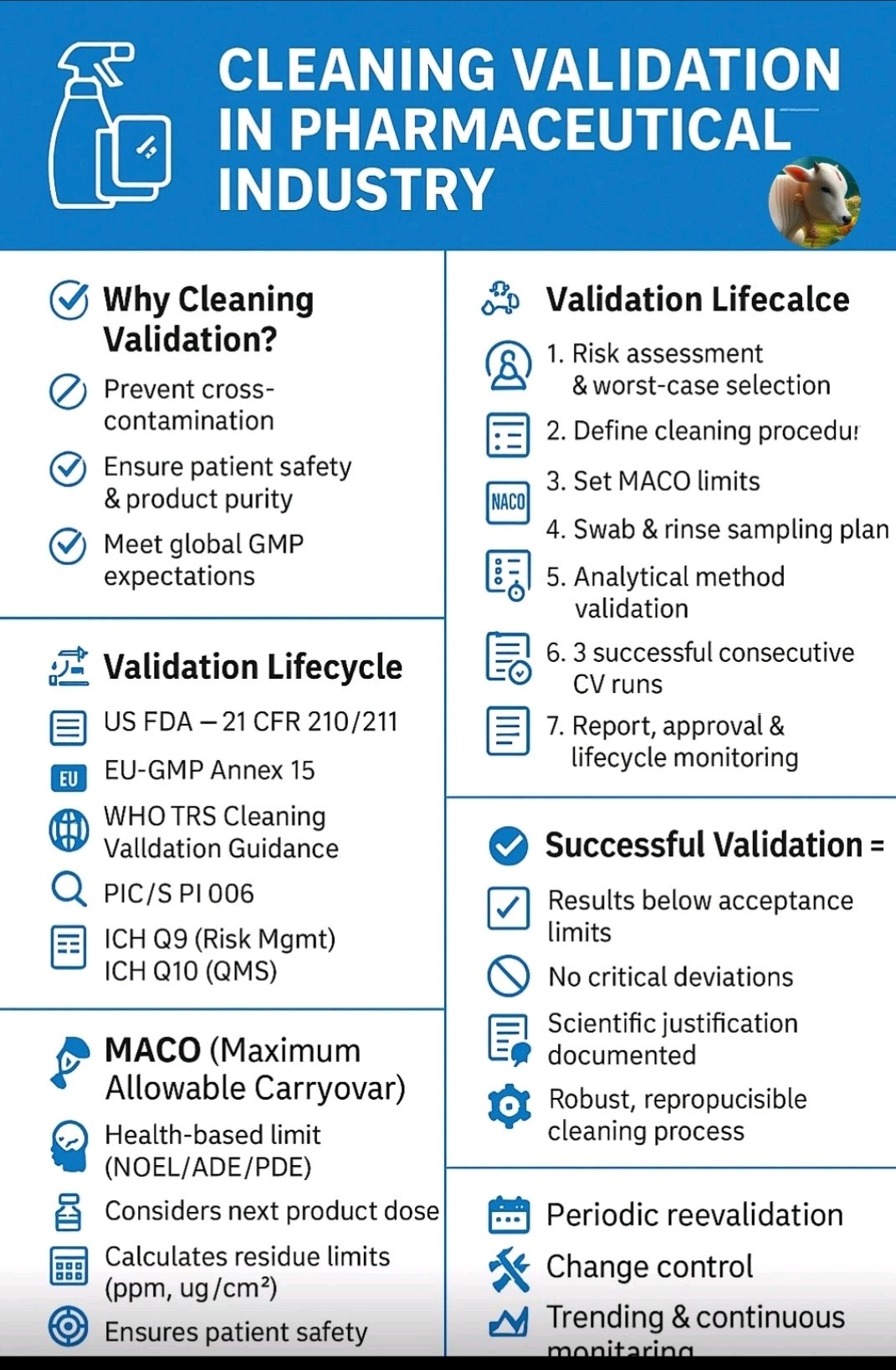
Cleaning validation guidelines provide a systematic approach to proving that cleaning procedures consistently and effectively remove product, cleaning agent, and microbial residues from equipment to acceptable levels. Key steps include creating a detailed validation protocol with a risk assessment, defining acceptance criteria, selecting worst-case products, establishing validated analytical methods, and performing sampling and testing (swab or rinse).