Stability Studies – an overview
- Stability: Fundamentals
The ability of a pharmaceutical product to retain its
properties within specified limits throughout its shelf life.
Aspects of stability that are to be considered include:
Physical, Chemical, Microbiological & Biopharmaceuticals.
It is It is a study on the evidence on how the quality of a drug substance or drug products varies with time under the influence of variety of environmental factors such as Temperature, Humidity and Light.
- Objective – why stability
- To provide evidence on how the quality of a drug substance / product varies with the influence of variety of environmental factors such as temperature, humidity & light
- To recommend storage conditions
- To recommend retest period
- To assign shelf life
- To review the product quality
- To fulfill the regulatory requirement for dossier submission
- Why is stability testing an important issue ?
- In order to demonstrate that:
- the clinical effect
- the patient safety
- the quality
of the drug is maintained during its maximal time of storage and intended use.
Starting a Study
Contents of a stability protocol
Setting the ‘start date’ for a stability study
Determining the ‘due dates’ for a stability study protocol The initial certificate of analysis at to for a stability study SOP Control
Format and layout of standard operating procedures Indexing procedure for stability studies
Index for stability sops
Study Parameters
Setting limits for check specifications in a stability study. Number and size of batches for stability testing.
Sampling
Number of samples required for performing stability tests Labeling of stability study samples
Storage configuration of samples in a stability environment
Storing the stability study samples under controlled conditions prior to analysis
Study Conditions
Intervals and climatic conditions for a development stability study Intervals and climatic conditions for a pivotal/bioequivalence stability study
Intervals and climatic conditions for a validation/pm stability study Placing the reference listed drug (RLB) on stability
Packaging procedures
Sampling and testing of pivotal batches – tablet & capsule dosage forms. Sampling and testing of pivotal batches – powder & syrups for reconstitution.
Container systems
Container-liner-closure systems for a stability study.
Certification of a container-liner-closure system.
Test methods
The control of analytical methods #’s and edition #’s in stability documentation
Test results
Reporting test results of a stability study.
Procedures for handling abnormal or OOS results in a stability study.
Audit and Review Raw Data
Auditing stability data in laboratory notebooks
Cross-referencing laboratory notebooks with computerized stability documentation
Chart Control
Recording stability study climatic conditions
Review and control of temperature and humidity recording charts
Validation and Sanitation
Periodic revalidation of climatic rooms and chambers
Sanitation and housekeeping requirements of climatic chambers
Corrective Action
Fault correcting procedures (after breakdowns) during a Stability Study Emergency procedures during a stability study
Stopping a Study
Conditions for stopping a stability study.
Self Inspection
Self inspection procedures in a stability department.
Job Description and Training
Job description of stability department personnel
Using stability SOPs and compliance program as stability training tools. The Do’s and Don’ts of a stability study – a department training tool.
Stability department compliance staff training
Reviewing Documentation
Review and auditing stability study documentation.
The layout and format of a regulatory stability report (a filed report)
Documentation requirements for a stability study – contents
of a stability dossier
Closing a Study
Accepting and signing-off a completed stability study.
Stability protocol – contents:
- Name of the product
- Batch size, type of batch and number of batches
- Source of API
- Type, size, source of containers and closures
- Storage condition
- Sampling schedule
- Container storage orientation
- Test parameters
- Test methods
- Acceptance criteria
- Stages of stability :
- Pre-formulation / pre-experimental stability.
- Formulation / experimental stability
- Pilot / post experimental stability
- Production / commercial stability
Stability studies during product life-cycle
 Guidelines for Stability Testing:
Guidelines for Stability Testing: - ICH guidelines on Stability testing of new drug substances
and drug products
- USFDA guidelines on Stability testing
- CPMP guidelines on stability
- WHO guidelines on stability testing of pharmaceutical products
Abbreviations – commonly used
- ICH: International Committee for harmonisation
- CPMP: Committee for proprietary Medicinal Products
- EMEA: European Agency for the evaluation of Medicinal products
- EFPIA: European Federation of Pharmaceutical Industries & associations
- FDA: Food and Drug Administration
- PhRMA: Pharmaceutical Research and Manufacturers of America
- MHLW: Ministry of Health, Labour Welfare
- JPMA: Japan Pharmaceutical Manufacturers Association
Global climatic zones:
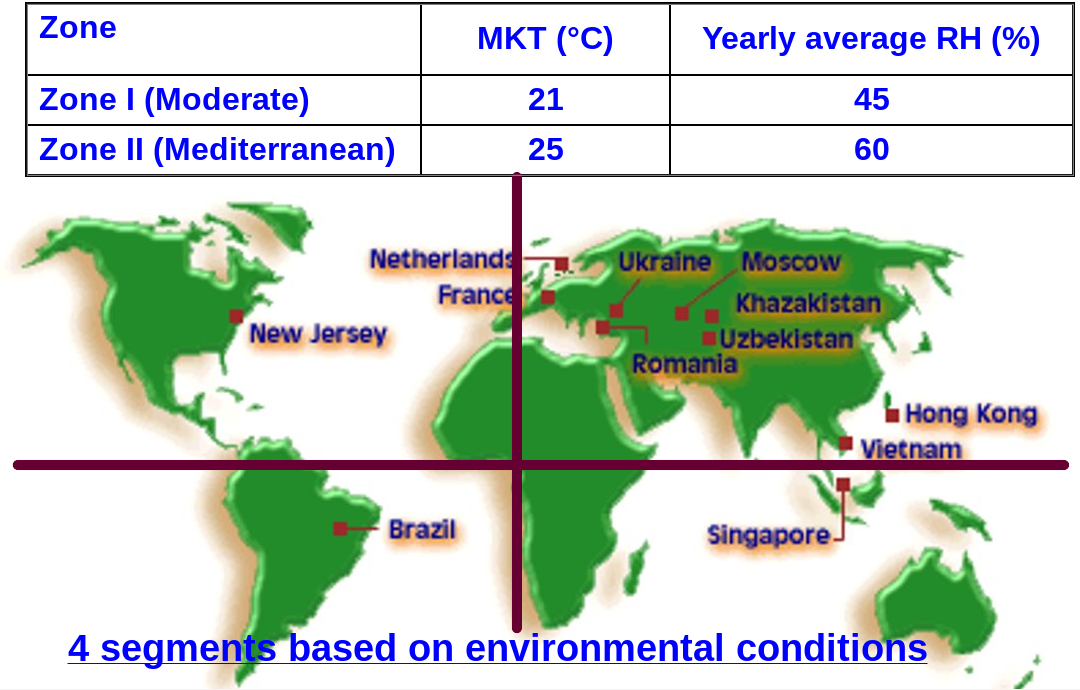 Distribution of nations into different climatic zones:
Distribution of nations into different climatic zones: Region Zones I & II Zones III & IV European All countries – American Chile, Canada, United States Brazil, Jamaica, Venezuela Asian China, Japan,
Turkey
India, Philippines,
Sri Lanka
African South Africa,
Zambia, Zimbabwe
Botswana, Ghana, Uganda Australian / Oceanic Australia, New Zealand Fiji, Papua – New Guinea Q1A : Stability Testing of New Drug Substances & Products.
Q1B : Stability Testing : Photostability testing of New Drug Substances & Products.
Q1C : Stability Testing for New Dosage Forms.
Q1D : Bracketing & Matrixing Designs for Stability Testing of Drug Substances & Products.
Q1E : Evaluation of Stability Data.
Q1F : Stability Data Package for Registration Applications in Climatic Zones III & IV.
Q3A : Impurities in New Drug Substances.
Q3B : Impurities in New Drug Products.
Q3C : Impurities: Guideline for Residual Solvents.
Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance).
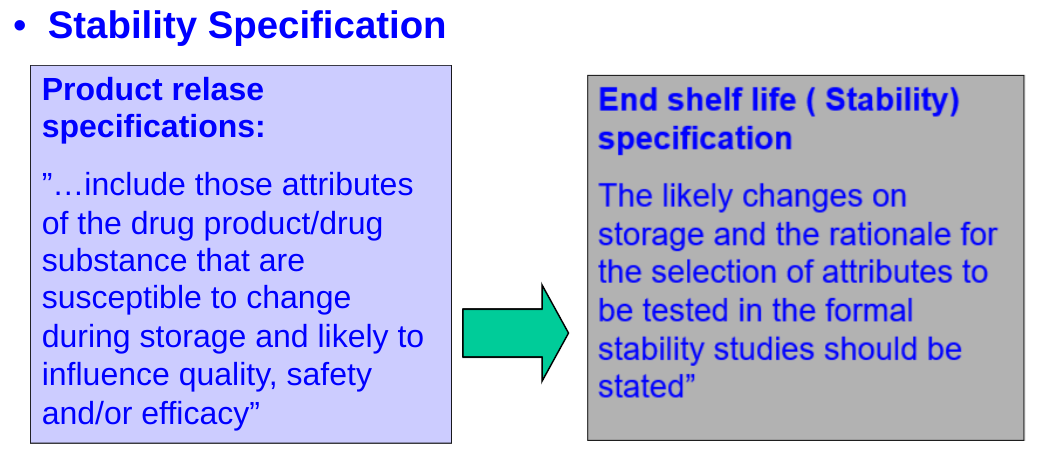
Specifications :
- List of tests.
- Testing procedure.
- Acceptance criteria (at the time of release / shelf life).
- Reference standards.
- In-process tests.
- Physical tests such as particle size distribution
- Parametric releases.
- Various decision trees
- Impurities
- Micro limits.
The testing should cover as appropriate : chemical, physical, biological & microbiological parameters. Validated analytical method should be adopted.
- Stability Testing Frequency
- Long Term : First year : Every 3 months.
Second year : Every 6 months.
Thereafter : Annually.
- Intermediate : 0, 3, 6, 9, 12 months.
- Accelerated : 0, 3, 6 months.
Storage Condition : General case:
Study Storage condition Minimum time period covered by data at submission Long Term 25 20C / 60 5% RH 12 months Intermediate 30 20C / 65 5% RH 6 months Accelerated 40 20C / 75 5% RH 6 months Any “significant change” occurs during 6 month accelerated study, additional testing at intermediate storage should be conducted. The initial application should include a minimum of 6 moths data from 12 month study of intermediate storage condition.
“Significant change” for a drug substance is failure to meet specification. For drug product, it is given separately.
Storage Condition :
Drug Substance / Drug Product intended for storage in refrigerator :
Study
Storage condition
Minimum time period covered
by data at submission
Long Term
5 30C
12 months
Accelerated
25 20C / 60 5% RH
6 months
If “significant change” occurs between 3 & 6 months of
accelerated study, data on long term study should be submitted.
If “significant change” occurs within 3 months of accelerated study, it is unnecessary to continue further testing.
iii. Storage Condition Drug Substance / Drug Product intended for storage in freezer :
Study
Storage condition
Minimum time period covered
by data at submission
Long Term
– 20 50C
12 months
There is no accelerated study for above case.
Drug substance intended for storage below – 20 0C should be treated case by case.
- COMMITMENT
- If the submission does not include stability data on production batches, a commitment should be made to place the first 3 production batches on long term studies during the proposed shelf life and accelerated studies for 6 months. Initial long-term data on primary batches may not cover the proposed shelf life granted at the time of approval.
- COMMITMENT
- Initial long-term data on primary batches may not cover the proposed shelf life granted at the time of approval.
- Commitment is made to continue the post approval studies in order to
firmly establish the shelf life.
- If the submission includes data from studies on less than 3 production batches, a commitment is made to continue the long term studies during the proposed shelf life and place additional production batches to make a total of at least 3 on long-term studies through the proposed shelf life.
- If the submission does not include stability data on production batches, a commitment should be made to place the first 3 production batches on long term studies during the proposed shelf life and accelerated studies for 6 months. Initial long-term data on primary batches may not cover the proposed shelf life granted at the time of approval.